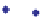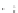Geology Reference
In-Depth Information
4.0
2.5
Shinn
et al
. (1989)
Lowenstam & Epstein (1957)
This study
3.5
2.0
3.0
1.5
2.5
1.0
2.0
1.5
0.5
1.0
0
0.5
30
32
34
36
38
40
42
44
Salinity (psu)
0
30
35
40
45
50
13
C of DIC and salinity for
samples collected in this study. There is no statistically
signifi cant correlation in these data between
Fig. 8.
Relationship between
Salinity (psu)
13
C of DIC
18
O and salinity for water
samples from GBB measured by Shinn
et al
. (1989),
Lowenstam & Epstein (1957) and this study. The line shows
a model result of the evaporation of a water body with an
initial salinity of 32 and
Fig. 7.
Relationship between
and salinity.
18
O of 0‰ using the equations
outlined by Gonfi antini (1986) and the model of Craig &
Gordon (1965).
between C and O found in modern sediments
collected from Belize and the Persian Gulf
(Gischler
et al.,
2007).
aragonite higher than 60%, where there is a strong
positive association between percentage aragonite
and
RELATIONSHIPS ON GREAT BAHAMA
BANK
13
C, and one at concentrations below 60%,
where there is apparently no correlation between
the percentage of aragonite and
Salinity and stable isotopes
13
C (Fig. 9b). This
apparent difference probably arises because there
are at least two sources of aragonite with very
different
There have been no systematic temporal or
spatial investigations of the water chemistry of
Great Bahama Bank, but the analyses that have
been made show waters increasing in salin-
ity as one progresses across Great Bahama Bank
from west to east (Black, 1933; Smith, 1940b;
Lowenstam & Epstein, 1957; Cloud Jr., 1962;
Shinn
et al.,
1989). Very close to Andros Island,
salinities have been shown to decrease as a result
of runoff (Cloud Jr., 1962).
Oxygen-isotopic analyses of the waters generally
show a weak positive relationship with salinity as
do the data measured in this study (Lowenstam &
Epstein, 1957; Shinn
et al.,
1989) (Fig. 7). However,
as a result of exchange between atmospheric
water vapour and the water on GBB, the maximum
13
C values on GBB. One source is the
calcareous green algae exhibiting high
13
C values,
mentioned above. The other source is the aragonite
produced by scleractinian corals and red calcare-
ous algae which possess much lower
13
C values
(
1 to +1‰) (Keith & Weber, 1965; Milliman, 1974;
Land, 1989). Hence, increasing the concentration
of aragonite by adding material derived from cor-
als and red calcareous algae will not result in a
corresponding
13
C increase and will not pro-
duce a correlation between the concentration of
aragonite and
13
C. A similar result was found at
Enewetok, with relationships between aragonite
and
13
C being either positive or negative depend-
ing upon the location sampled (Weber & Schmalz,
1968). Another signifi cant study which examined
a much larger spatial area was that by Dix
et al
.
(2005). Here workers examined approximately
100 samples collected over an approximate
600 km
2
area on the North West Australian Shelf
and also identifi ed a positive relationship between
the percentage of aragonite and
18
O that can be attained is limited (Lloyd, 1964;
Craig & Gordon, 1965; Gonfi antini, 1986) and
therefore there is no clear relationship between
salinity and
18
O value
measured in this study was ~+2.8‰ at a salin-
ity of 35.75. Waters with higher salinity values
had actually lower
18
O. In fact the maximum
18
O values. The
D data also
show clear evidence of a strong evaporative trend
(Fig. 10) with original source water close to 0‰.
As the samples were collected over a four-year
time period during which the salinity and
13
C, again pro-
duced by a mixing between isotopically positive
aragonite and more depleted LMC. A similar expla-
nation has been invoked for different correlations
18
O of