Geology Reference
In-Depth Information
(a)
3.5
Calcareous
green algae
3.0
2.5
2.0
Calcareous
red algae
1.5
1.0
Corals
0.5
Slope
Rim
Reef flat
Lagoon
Slope
0
1
3
5
7
9
11
13
15
17
19
21
23
25
27
29
31
33
Sampling station
13
C data from
Heron Island, Australia showing
the
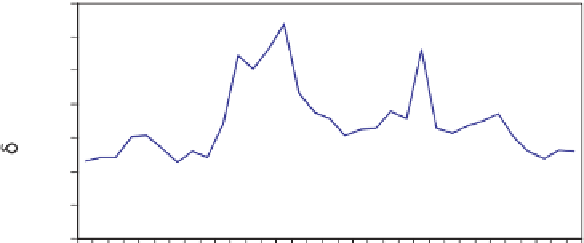
Fig. 9.
(a) Sediment
(b)
3.5
13
C values in a south to north
transect (low numbers to high num-
bers) across the reef. See Weber &
Woodhead (1969) for sample loca-
tion. Changes in
3.0
2.5
13
C correspond
to changes in facies with elevated
values corresponding to areas domi-
nated by calcareous green algae and
lower values in areas where there are
abundant red algae and scleractinian
corals (Weber & Woodhead, 1969).
(b) Sediment
2.0
1.5
1.0
0.5
0
13
C data shown in (a)
correlated with the percentage of
aragonite.
0
20
40
60
80
100
Aragonite (%)
the water varied as a result of weather conditions,
it is not possible to combine the data and make
a generalization about the distribution of
40
30
18
O on
GBB. However, it is probable that waters further
from the margin might have elevated
20
10
0
18
O values
on a consistent basis. Hence carbonates formed
further from the margin might also be expected to
have higher
−
10
−
20
−
30
18
O values than carbonates found on
or near the margin.
Previous studies on the
−
40
−
6
−
5
−
4
−
3
−
2
−
1
0
1
2
3
13
C of waters on GBB
are limited to one, which showed large ranges
in
18
O (‰)
13
C ranging from ~
5 to +1‰ (Patterson &
Walter, 1994). It was suggested that these varia-
tions were related to oxidation of organic
material, photosynthesis and precipitation of ara-
gonite and LMC. The
18
O and
Fig. 10.
Relationship between
D in the data
measured in this study relative to the meteoric water line
(dashed line). The solid line shows the evaporative trend of
a water body using the same conditions as used in Fig. 7.
13
C of the DIC measured
in this study were signifi cantly higher and var-
ied from ~ +0.5 to +2.5‰ (Fig. 8). In no instance
over fi ve years of analyses were waters with
the HCO
3
pool as CO
2
is produced during the
precipitation of CaCO
3
. Photosynthesis by the
abundant algae and seagrasses on GBB prefer-
entially removes the lighter isotope of carbon,
causing the residual bicarbonate pool to become
enriched in the heavier isotope of carbon,
while precipitation of calcium carbonate lowers
the pH, converting HCO
3
into CO
2
. As there is
an isotopic fractionation of approximately 8%
13
C values less than +0.5‰ recorded. The lower
values measured in this study are typical of
open marine values (Weber & Woodhead, 1971;
Kroopnick, 1974; Swart
et al.,
2005), while the
higher ones suggest both fractionation of CO
2
during photosynthesis and/or fractionation of