Environmental Engineering Reference
In-Depth Information
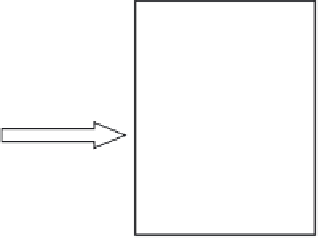
Addition of sulfur
source
Solution of cadmium source
in capping agent
Nanoparticles coated
in capping agent
Figure 2.17
The preparation of a nanoparticle dispersion using the arrested precipitation
method.
of stable suspensions, where as the micelle and arrested precipitation methods will
results in stable dispersions under various conditions but with the added complexity
of at least one further component to consider; the capping agent or surfactant.
Furthermore, unless care is exercised in the purifi cation of the materials there is
likely to be contamination of the particle by either by products from synthesis or
unreacted starting materials. Some of the starting materials for certain nanoparti-
cles are exceptionally toxic.
It is important to consider that the formation of nanoparticles by the bottom up
approach is a dynamic process and that the surface of the nanoparticle cannot be
considered to be unreactive. In fact, various processes are known to affect particle
form long after the nanoparticles themselves have been prepared. There are two
important factors. One is Oswalds ripening, which results in the sacrifi cial dissolu-
tion of small particles in favour of growth of the larger particles with lower surface
energies. The rate of such a process is related to the distribution of particle sizes as
well as the specifi c chemistry of the particles themselves. The other important factor
is aggregation of the particles to form larger particles. These may become sintered
into a polycrystalline larger particle. These processes have much to do with the fate
of nanoparticles and are discussed further in Chapter 3.
A brief overview of the types of methods used to prepare a range of nanomateri-
als is given below. These examples are by no means exhaustive. However, they will
give an overview of the general methods, reagent and so on that may be used in
preparing some nanomaterials.
2.5.8
Metal Nanoparticles
Metal nanoparticles are perhaps the earliest forms of nanoparticles prepared by
man. The general method of preparation has changed little and generally relies on
the reduction of a dissolved metal salt in the presence of a suitable capping agent
or surfactant. The exact method employed depends on the metal. For example, gold














Search WWH ::

Custom Search