Environmental Engineering Reference
In-Depth Information
Microemulsion of CdCl
2
Microemulsion of Na
2
S
Mixing of Solutions
Nanoparticles of CdS are formed in micelles
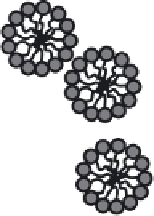
Figure 2.16
Preparation of nanoparticles of cadmium sulfi de in a microemulsion.
collide, combine and reform very rapidly, resulting in the mixing of the core solu-
tions whist retaining the micelle structure. The formation of the nanoparticle is then
constrained by the micelle itself. This type of approach has been applied to both
oil - in - water and water - in - oil emulsions as is discussed later.
Arrested p recipitation
There are some similarities between arrested precipitation and micelle encapsula-
tion, notably that both employ a surfactant to control the particle size. However,
whilst in the micelle method the surfactant forms structures which contain the
nanoparticles, in arrested precipitation the surfactant simply partitions to the
surface of the particle as the particle forms. Micelles are very unstable at elevated
temperatures (
50 °C) whereas arrested precipitation methods have been applied
at temperatures exceeding 300 °C. During an arrested precipitation reaction (Figure
2.17) generally a solution of one reagent is heated to the required temperature and
then treated with a solution of the second reagent. It is not uncommon to conduct
the whole reaction in the surfactant or capping agent alone with out any further
solvent. The fi nal particles are washed to removes some of the excess capping agent.
>
The overall outcome of this is that fl ame pyrolysis type preparation methods will
result in very pure materials, but with no surface coating to ensure the formation













Search WWH ::

Custom Search