Environmental Engineering Reference
In-Depth Information
Figure 3.8 Plots of the heights of the steps in Fig. 3.5 divided by the electron charge in order
to get a potential. Via the relations in Fig. 3.7, these steps are all functions of E
O
. On the vertical
axis is the highest potential at which a step in the ORR is downhill in free energy, depicted as a
function of the binding of O
. The step that first becomes uphill in free energy defines U
ORR
Max
(DE
O
)U. Steps 1 and 4 (lines labeled DG
1
and DG
4
, respectively) define the lower “volcano”
and thereby U
ORR
Max
(DE
O
). Pt is the pure metal closest to the top.
different intermediates, it is possible to obtain a quantitative version of the Sabatier
principle, stating at which value of the “descriptor,” in this case E
O
, the best tradeoff
is obtained. We note that the binding of OH
would have served as an equally valid
descriptor.
Whereas U
ORR
Max
is relatively easy to determine from the calculated binding energies,
it is not easy to measure experimentally, since the measured potentials are always
related to a specific current. Therefore, in order to compare directly with experiment,
we have to calculate polarization curves, i.e., the current. The link between U
ORR
Max
and
the current is the Tafel equation,
j
c
¼
j
0
exp(ahe
=
k
B
T)
(3
:
19)
where j
c
is the cathode current density, j
0
is the exchange current, his the overpoten-
tial, and ais the transfer coefficient. We will here only consider a¼ 1, but the model
could be constructed for other values of a. The overpotential is written as h; U
0
2 U
in order to rewrite the Tafel equation as
¼
j
limit
exp
a(U
Max
j
c
¼
j
0
exp
a(eU
0
eU)
k
B
T
ORR
eU)
k
B
T
(3
:
20)


















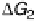









Search WWH ::

Custom Search