Environmental Engineering Reference
In-Depth Information
Figure 17.13 (a) Representation of the crystallographic structure of the [NiFe]-hydrogenase
from the sulfate-reducing bacterium Desulfovibrio fructosovorans (PDB file 1YRQ). A
number of hydrogenases have been shown to be active catalysts for both H
þ
reduction and
H
2
oxidation when adsorbed on a graphite electrode (for a review, see Vincent et al. [2007]).
The small protein subunit incorporates three FeS clusters shown in gray spheres; the large
protein subunit incorporates the NiFe active site, also shown in gray spheres. The protein is rep-
resented as thin gray sticks.
although they arose as a result of separate evolutionary pathways [Fontecilla-Camps
et al., 2007]. The [FeFe]-hydrogenases can also be very active electrocatalysts (for a
review, see Vincent et al. [2007]), but we focus in this chapter on catalysis by the
[NiFe]-enzymes, which tend to be more robust in air.
In biology, the “electrical wiring” by a relay chain of FeS clusters between the
active site and the surface of the protein allows electrons from H
2
oxidation to be
taken up by cellular electron acceptors (often heme-containing oxidases; see
Fig. 17.2), but this feature of hydrogenases also makes them well equipped for
direct coupling to an electrode surface. The following sections describe electrochem-
istry at graphite electrodes modified with hydrogenase, and provide examples of the
use of these electrodes in fuel cell catalysis.


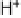









Search WWH ::

Custom Search