Environmental Engineering Reference
In-Depth Information
Figure 17.14 Cyclic voltammograms recorded at 1 V s
21
at a PGE RDE rotating at 2500 rev
min
21
) modified by adsorption of a submonolayer film of [NiFe]-hydrogenase from the purple
photosynthetic sulfur bacterium Allochromatium vinosum in buffered aqueous solution at pH
7.0 under an atmosphere of H
2
(1 bar). Reprinted with permission from L´ger et al., 2002.
Copyright (2002) American Chemical Society.
Electrocatalysis by a single layer of [NiFe]-hydrogenase on a PGE RDE is shown in
Fig. 17.14 [L´ger et al., 2002]. The film of enzyme was prepared by adsorption from a
dilute solution of Allochromatium vinosum hydrogenase and a polyamine co-adsorbate,
polymyxin, over a period of about 30 minutes, during which the electrode potential was
cycled between 20.58 and 0.24 V versus SHE. Figure 17.14 shows catalytic cyclic
voltammograms that result when the electrode is placed in enzyme-free, neutral aqueous
solution in contact with 1 bar H
2
. A positive current above about 20.4 V corresponds
to H
2
oxidation by the enzyme and direct transfer of electrons from the enzyme to the
electrode, i.e., with no external mediation. As the temperature is raised, the catalytic
current increases, reaching over 2 mA cm
22
at 60 8C[L´ger et al., 2002].
The shape of the hydrogenase catalytic voltammograms shown in Fig. 17.14 also
changes as the temperature is raised. At 10 8C, the current tends towards a plateau at
high overpotential as catalysis becomes limited by the inherent turnover frequency of
the enzyme, but at higher temperatures, the current continues to increase linearly with
electrochemical driving force. This has been attributed to a range of different


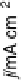








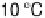
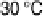




















Search WWH ::

Custom Search