Environmental Engineering Reference
In-Depth Information
charge
q. Thus, q plays the role of a reaction coordinate in the sense of Kramers
[1940] theory; all other modes form part of the heat bath and give rise to friction
effects, which will be discussed below. For simplicity, we shall drop the tilde hence-
forth, and assume that q is normalized.
The energy of the system can be calculated as a function of q by Green function
techniques [Davison and Sulston, 2006]. For this purpose, it is convenient to gather
the terms containing n
a
in the Hamiltonian and introduce an electronic energy that
depends on the solvent configuration through
1
a
(q)
¼
1
a
2lq
(2
:
5)
The density of states of the reactant is then given by [Schmickler, 1986]
r(1)
¼
1
p
D(1)
[1
1
a
(q)
L(1)]
2
þ
D(1)
2
(2
:
6)
where the so-called chemisorption functions [Davison and Sulston, 2006] are
D(1)
¼
p
X
k
L(1)
¼
P
X
k
j
V
ak
j
2
1
1
k
j
V
ak
j
2
d(1
1
k
),
(2
:
7)
P denotes the principal value.
In outer sphere electron transfer, the reactant is not adsorbed; therefore, the inter-
action with the metal is not as strong as with the catalytic reactions discussed below.
Hence, the details of the metal band structure are not important, and the coupling
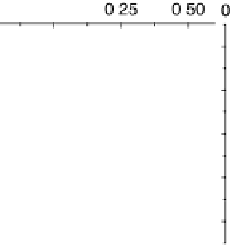
D(1) can be taken as constant. This is the so-called wide band approximation, because
it corresponds to the interaction with a wide, structureless band on the metal. In this
approximation, the function L(1) vanishes, and the reactant's density of states takes
the form of a Lorentzian. The situation is illustrated in Fig. 2.3.
Figure 2.3
The wide band approximation.





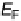
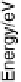
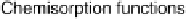
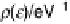
























Search WWH ::

Custom Search