Environmental Engineering Reference
In-Depth Information
show extensive adsorption of acetic acid, which increases at more positive poten-
tials and maximizes at the onset of anodic oxide formation. This is what we con-
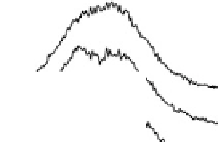
firmed here (Fig. 12.15). A single band near 1420 cm
21
was found [Corrigan
et al., 1988] that is due to adsorption of acetic acid on Pt, exactly as in
Fig. 12.15. The 1420 cm
21
feature was consistent with a symmetric carboxylate
stretching mode, with both carboxylate oxygens oriented towards the metal sur-
face. We then concluded [Corrigan et al., 1988] that the most likely modes of
acetic acid adsorption involve hydrogen bonding between the carboxylate oxy-
gens and inner layer water molecules or that the adsorption occurs by self-associ-
ation to form a dimer or chain structure. While in this brief study we cannot
confirm the nature of surface coordination and orientation of the adsorbed
acetic acid species, it is tempting to conclude from the coincidence between
the spectral data in [Corrigan et al., 1988] and in Fig. 12.15 that a similar surface
structure is found, including the role of Pt oxides in guiding surface concen-
tration of the reversibly adsorbed acetic acid adsorbate.
12.4.6 Chemisorption of CO on Nanoparticle Pt Electrodes
Pt nanoparticles, made of either pure or alloyed Pt, are well known as anodes and
cathodes in hydrogen and methanol fuel cells [Vielstich et al., 2003]. Using
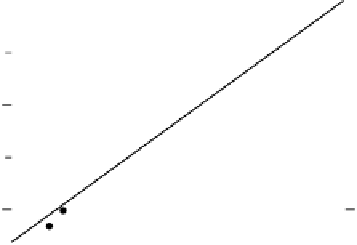
Figure 12.16 Potential dependent SFG spectra (a) and the Stark tuning plot (b) from chemi-
sorbed CO on Pt nanoparticles in a CO-saturated 0.1 M H
2
SO
4
electrolyte. Each spectrum was
acquired for 10 s (forward scan data only are shown). The potential was scanned from 20.20 to
0.70 V (vs. Ag/AgCl) at 1 mV/s. Pt nanoparticles were of approximately 7 nm size, and were
immobilized on an Au disk.



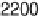




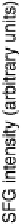









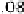










































Search WWH ::

Custom Search