Environmental Engineering Reference
In-Depth Information
For this reason, the development of cathode electrocatalysts insensitive to alcohols
is of great interest. Among such catalysts, metal macrocycles, and most particularly
iron phthalocyanines (FePcs) and porphyrins (FePPs), have been extensively studied
[Lalande et al., 1995, 1996; Zagal et al., 1980; Coutanceau et al., 1994, 1995]. These
catalysts were shown to be totally insensitive to the presence of methanol [Jiang and
Chu, 2000; Convert et al., 2001; Baranton et al., 2005], and present a good alternative
to Pt, which is a rare and expensive material. FePc/C catalysts displayed better cata-
lytic activity with regard to the ORR than Pt in the presence of methanol (Fig. 11.13),
and very good stability when used under working conditions close to that of a DMFC
at room temperature [Baranton et al., 2005] (Fig. 11.14). It was proposed that using a
solid proton exchange membrane as electrolyte, avoiding the presence of “free” pro-
tons as in liquid acid electrolytes, avoids demetalation of the macrocycle [Baranton
et al., 2005]. These characteristics made FePc a good candidate for a cathode catalyst,
at least for a fuel cell working at or close to room temperature, for example for mini and
micro fuel cells developed for portable applications [Lu et al., 2004; Yamazaki, 2004].
However, FePc catalysts do not match Pt catalyst performances in methanol-free
oxygen-saturated solutions [Baranton et al., 2005]. It is therefore very important to
obtain a better understanding of the oxygen reduction mechanism at such macrocyclic
catalysts in order to propose some ways to improve their activity and their selectivity.
For example, a co-facial structure (Fig. 11.15) of the macrocycles with a metal - metal
distance close to 0.4 nm seems to improve the electroactivity with regard to oxygen
reduction as well as the selectivity with regard to water formation via a four-electron
process [Collman et al., 1980; Baranton et al., 2006].
For example, the a- and b-phases of FePc, which correspond to co-facial structures
of the macrocycles, were studied and compared. The structure of MPcs (M ¼ metal)
Figure 11.13 Linear cyclic voltammograms of different carbon-supported catalysts recorded
in an O
2
-saturated electrolyte (0.5 M H
2
SO
4
): (1) Pt/C catalyst; (2) Pt/C catalyst in the presence
of 1.0 M methanol; (3) FePc/C catalyst; (4) FePc/C catalyst in the presence of 1.0 M methanol
(temperature 20 8C, scan rate 5 mV s
21
, rotation speed 2500 rev min
21
).

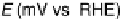

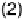

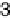















Search WWH ::

Custom Search