Environmental Engineering Reference
In-Depth Information
effects should enhance the reaction rates of oxygen adsorption and of O - O bond
breaking during the reduction reaction. For example, the lattice parameter a
0
in the
case of cubic Pt-X (X ¼ Fe, Co, Ni) decreases with increasing content of the alloying
component X, leading to a variation in catalytic behavior. In the case of Pt-Ni alloys,
the maximum electrochemical activity for ORR is obtained with 30 at% Ni [Toda
et al., 1999a, b]. The presence of highly uncoordinated atoms is also very important,
as shown by Lemire and co-workers for CO adsorption at Au nanoparticles [Lemire
et al., 2004]. Finally, some authors have proposed that the role of the foreign metal
is to protect the Pt surface from oxidation, by the presence of more easily oxidizable
species [Shukla et al., 2001].
Such bimetallic alloys display higher tolerance to the presence of methanol, as
shown in Fig. 11.12, where Pt-Cr/C is compared with Pt/C. However, an increase
in alcohol concentration leads to a decrease in the tolerance of the catalyst [Koffi
et al., 2005; Coutanceau et al., 2006]. Low power densities are currently obtained in
DMFCs working at low temperature [Hogarth and Ralph, 2002] because it is difficult
to activate the oxidation reaction of the alcohol and the reduction reaction of molecular
oxygen at room temperature. To counterbalance the loss of performance of the cell due
to low reaction rates, the membrane thickness can be reduced in order to increase its
conductance [Shen et al., 2004]. As a result, methanol crossover is strongly increased.
This could be detrimental to the fuel cell's electrical performance, as methanol acts as
a poison for conventional Pt-based catalysts present in fuel cell cathodes, especially in
the case of mini or micro fuel cell applications, where high methanol concentrations
are required (5 - 10 M).
Figure 11.12 Linear cyclic voltammograms of carbon-supported nanosized Pt and Pt-Cr
alloy catalysts with different atomic ratios (prepared using the carbonyl route [Yang et al.,
2004]) recorded in 0.5 M HClO
4
þ
0.5 M CH
3
OH saturated with pure oxygen at a scan rate
of 5 mV s
21
and a rotation speed of 2000 rev min
21
. ——, Pt/C;----,Pt
0.7
Cr
0.3
/C; —
- — -, Pt
0.5
Cr
0.5
/C.


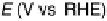
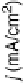


















Search WWH ::

Custom Search