Environmental Engineering Reference
In-Depth Information
Figure 10.6 Decomposition of Pt4f
7/2
spectra (open circles) into surface and bulk core levels
(solid and dashed lines), respectively, for stabilized Pt
58
Co
42
alloy (a) before and (b) after CO
adsorption. (From Wakisaka et al. [2006], reproduced by permission of the American Chemical
Society.)
Figure 10.6 shows CL spectra of Pt4f
7/2
for stabilized Pt-Co with and without
CO
ad
. The CO adsorption induced both a shift in the Pt4f
7/2
CL to higher binding
energy and an increase in the full width at half maximum (FWHM). Such changes
can be explained by surface core level (SCL) shifts of Pt4f
7/2
by CO
ad
, whereas the
bulk CL is not affected by CO
ad
. In order to extract the change in SCL shift
(DSCLS) by CO
ad
, we decomposed the Pt4f
7/2
spectra into two components: the
bulk CL and SCL. It was found that the value of DSCLS decreased in the order
pure Pt . stabilized Pt-Co . Pt-Ru.
The CO adsorption energy (the energy required to break the Pt - CO bond),
E
ads
(CO/Pt), was related to DSCLS by the following equation [Treglia et al., 1981;


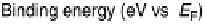
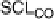

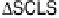

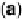











Search WWH ::

Custom Search