Environmental Engineering Reference
In-Depth Information
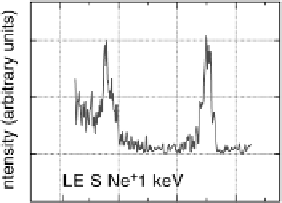
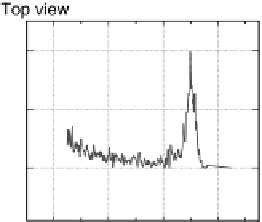
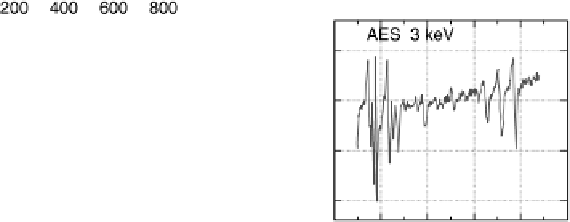
Figure 8.11 (a, b) PtCo surface after exposure to H
2
O: (a) Auger spectra reveal the formation
of stable oxides (black spheres); (b) LEIS spectra confirm that the Co surface concentration
remains the same upon transfer to UHV. (c, d) PtCo surface after exposure to 0.1 M HClO
4
:
(c) Auger spectra show a decrease in intensity of the Co peaks; (d) LEIS spectra reveal that
the PtCo surface contains only Pt atoms in the topmost atomic layer after exposure to the elec-
trolyte, surface Co atoms are being dissolved forming a Pt-skeleton surface. (Reprinted with per-
mission from Stamenkovic et al. [2006a]. Copyright 2006. The American Chemical Society.)
(electro)chemical environment. Overall, we concluded that in both the Pt-skin and Pt-
skeleton surfaces, the pure Pt outermost layer protects the subsurface transition metals
atoms from (further) dissolution.
This allows a direct influence of the alloying component on the electronic proper-
ties of these unique Pt near-surface formations from subsurface layers, which is the
crucial difference in these materials. In addition, the electronic and geometric struc-
tures of skin and skeleton were found to be different; for example, the skin surface
is “smoother” and the d-band center position with respect to the metallic Fermi
level is downshifted for skin surfaces (Fig. 8.12) [Stamenkovic et al., 2006a] owing
to the higher content of non-Pt atoms in the second layer. On both types of surface,
the relationship between the specific activity for the oxygen reduction reaction
(ORR) and the d-band center position exhibits a volcano-shape, with the maximum


















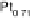
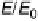

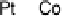

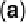
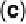
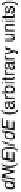
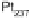
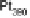

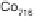
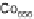



























































Search WWH ::

Custom Search