Environmental Engineering Reference
In-Depth Information
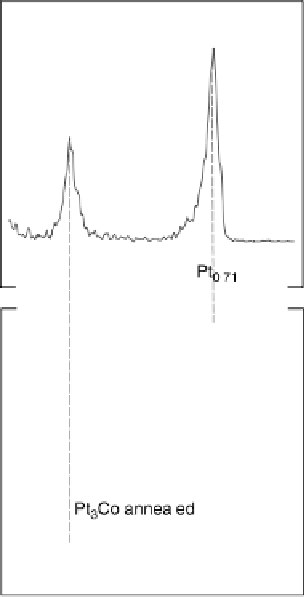
Figure 8.10 LEIS spectra of Pt
3
Co: (a) lightly sputtered surface with 0.5 keV Ar
þ
ions; (b)
annealed surface at 1000K. (Reprinted with permission from Stamenkovic et al. [2002].
Copyright 2002. The American Chemical Society.)
of surface Co atoms was revealed by the LEIS spectra shown in Fig. 8.11d, while Co
atoms are stable and covered with oxides (Fig. 8.11a, b), i.e., upon exposure of a PtCo
surface to an acidic solution, Co surface atoms are instantaneously dissolved from the
near-surface layers. The remaining surface, which consists only of Pt atoms we term a
Pt-skeleton. This type of surface had been described previously in the literature by
Watanabe and co-workers [Toda et al., 1999], but it was designated as a skin of Pt
with thickness of 3 - 5 atomic layers determined by X-ray photoelectron spectroscopy
(XPS) measurements. The conclusions drawn from this study are, however, equally
valid for the Pt
3
Co, Pt
3
Fe, Pt
3
Ni, Pt
3
Ti, and Pt
3
V sputtered surfaces. In each case,
whenever transition metal atoms are exposed to the acidic environment, non-Pt
atoms are dissolved, forming a Pt-skeleton surface. There may be some differences
between the alloys in the depth profile of transition metal atoms in the subsurface
layers, but that determination was not performed on polycrystalline systems. The
same set of ex situ analyses was performed on the Pt-skin surfaces formed over all
three Pt
3
M alloys, showing that the Pt-skin surface is a stable formation in an
























Search WWH ::

Custom Search