Environmental Engineering Reference
In-Depth Information
The same approach was used for the Pt
3
Sn(111) surface exposed to an electroche-
mical environment, where the oxygenated species are assumed to be OH
2
. The exist-
ence of two types of Sn sites (oxide-covered and oxide-free) causes compression of
CO
ad
molecules, resulting in higher local CO coverage, blue-shifted CO frequencies
due to enhanced dipole - dipole coupling, and onset of CO oxidation at 0.1 V.
Therefore, the oxidative removal of CO
ad
on Pt
3
Sn(111) at low potentials (based on
CO
2
production in Fig. 8.9 at about 0.1 V) proceeds on the surface sites where local
(microscopic) CO
ad
coverage remains high and thus CO
ad
is weakly adsorbed on Pt.
In contrast to the polarization curves obtained from rotating disk electrode measure-
ments [Gasteiger et al., 1996], spectroscopic data unambiguously show the true
onset potential for CO oxidation. This, in turn, emphasizes that classical electrochemi-
cal methods are not capable of measuring the reaction rates with very low turnover
frequency (TOF), such as the oxidative removal of CO
ad
from the Pt
3
Sn(111) surface
at low overpotential. Nevertheless, linking the microscopic and macroscopic levels of
characterization, the so-called weakly adsorbed (CO
ad
) [Markovic et al., 1999] state on
this surface may correspond microscopically to the formation of disordered but com-
pressed CO
ad
patches with a characteristic high frequency (2090 cm
21
) for atop CO
L
.
The remaining fraction of CO
L
with frequency of 2077 cm
21
may correspond to more
strongly adsorbed CO
ad
, which requires higher overpotentials to be oxidized. For E .
0.25 V, a pair of bands is transformed into a single peak shifted towards 2077 cm
21
(Fig. 8.8b). This transition is accompanied by the appearance of a bridge-bonded
CO
B
stretching band (centered at about 1820 cm
21
). As noted by many authors
[Lebedeva et al., 2000; Markovic and Ross, 2002], the oxidation of CO
ad
occurs
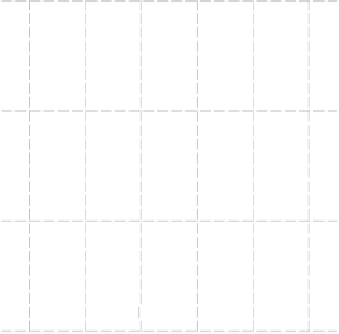
Figure 8.9 Polarization curves for a PtSn/C catalyst recorded by a rotating disk electrode in
0.5 M H
2
SO
4
saturated with either pure hydrogen, a H
2
/2% CO mixture, and pure CO (the
arrow points to the onset of CO oxidation) at 60 8Cwith1mV/s and 2500 rev/min; the dashed
curve is the cyclic voltammogram (in arbitrary units) in an argon-purged solution at 60 8Cwith
50 mV/s. (Reprinted with permission from Arenz et al. [2005]. Copyright 2005. Elsevier.)



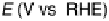
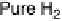
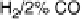
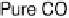



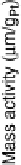
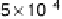
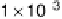


























Search WWH ::

Custom Search