Environmental Engineering Reference
In-Depth Information
surface structure and composition previously determined under UHV conditions were
stable in an electrochemical environment.
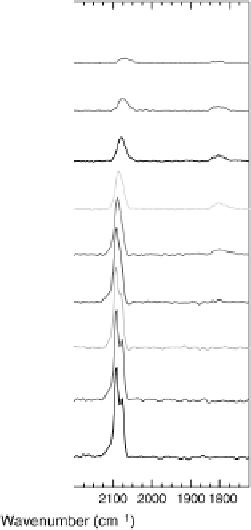
In addition, the properties of adsorbed CO were examined by in situ IRAS, and the
origin of the high catalytic activity was fully explained in combination with ex situ
UHV measurements (Fig. 8.8). In contrast to the near-invariant bands of atop CO
ad
on Pt(111) [Markovic et al., 2002], changes in the band shape (splitting of the
band) and frequency are clearly visible on the Pt
3
Sn(111) surface in the same potential
range. The splitting and the early onset of CO electro-oxidation have been explained
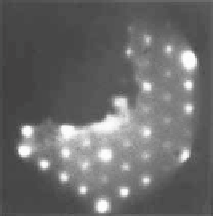
by the presence of oxygenated species on every second Sn atom, which was first con-
firmed by ex situ LEED, i.e., a sharp p(4
4) pattern was obtained by LEED on a
Pt
3
Sn(111) surface previously exposed to oxygen at 4
10
28
Torr at 300 8C
(Fig. 8.8c, d), having a Sn/Pt Auger electron spectroscopy (AES) ratio of 4.8 com-
pared with 4.5 for the p(2
2) pattern [Stamenkovic et al., 2003]. These results
also indicate that chemisorption of oxygen occurs without changing the underlying
structure or composition of the Pt
3
Sn(111) alloy surface.
Figure 8.8 Series of infrared spectra during (a) CO
2
production and (b) progressive oxidation of
CO
ad
on Pt
3
Sn(111) in 0.5 M H
2
SO
4
saturated with CO; each spectrum was accumulated from 50
interferometers at the potential indicated. (c, d) LEED pattern and schematic representation of the
p(4
4) structure observed on Pt
3
Sn(111) after exposing the surface to O
2
and electrolyte. The
gray circles are Pt surface atoms, the black circles are Sn atoms covered with OH, and the dotted
circles are Sn atoms that are chemically different from Sn atoms modified with OH. (Reprinted
with permission from Stamenkovic et al. [2003]. Copyright 1999. The American Chemical Society.)
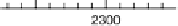


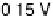
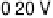

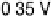
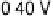






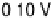


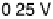


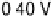
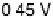


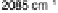
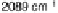
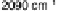

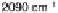


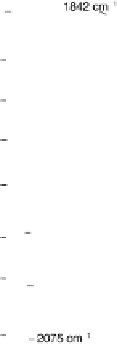
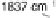
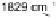



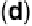
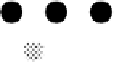




















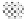































































































Search WWH ::

Custom Search