Environmental Engineering Reference
In-Depth Information
key requirement for understating reactivity of the electrochemical interface. In situ
infrared reflection absorption spectroscopy (IRAS) in a thin liquid film cell mode
has been successfully applied for characterization of electrified solid - liquid interfaces
[Iwasita and Nart, 1997].
This method provides the unique ability to follow changes at a molecular level
during the course of reaction. For instance, for each potential change, characteristic
infrared spectra can be obtained, and therefore the surface electrochemistry of reac-
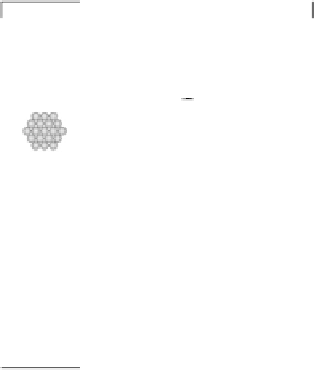
tants, products, and spectator molecular species can be followed. Figure 8.6 shows
spectra of adsorbed CO on Pt(111) and Au(hkl) surfaces obtained in an infrared cell
that has been designed in our laboratory. Besides the possibility of working under
temperature-controlled conditions, this configuration offers the opportunity to sub-
stantially decrease the recording time for each potential step. This has led to the devel-
opment of a so-called rapid scan mode of operation, which delivers a continuous mode
for recording of spectra for potential sweep rates between 1 and 10 mV/s.
For fundamental studies of nanoparticles in IRAS measurements, a very important
issue is how to attach metal nanoparticles onto a conducting substrate without chan-
ging their physical and chemical properties. Recently, we have developed a new
method for anchoring metallic nanoparticles on reflective substrates of gold and/or
glassy carbon, which we have termed a temperature-induced deposition (TID)
method [Stamenkovic et al., 2004]. A key advantage of this method is that the catalysts
Figure 8.6 (a) Infrared spectra of adsorbed CO on Pt(111) and Au(hkl) surfaces obtained in a
spectroelectrochemical cell (b) for in situ IRAS measurements at elevated temperatures (the
initial design of the cell was described by Iwasita [1997]): GI, gas inlet (bubbler); TP, thermo-
couple; WE, working electrode; CH, cartridge heate; CE, counter-electrode; RE, reference elec-
trode; CaF
2
, CaF
2
prism. (Reprinted with permission from Stamenkovic et al. [2004]. Copyright
2004. The American Chemical Society.)



















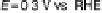

























































Search WWH ::

Custom Search