Environmental Engineering Reference
In-Depth Information
Figure 7.12 CO stripping experiment on a Pt(775) electrode decorated with different ada-
toms, as labelled, in 0.1 M HClO
4
. The blank voltammogram is also included for comparison.
Sweep rate: 50 mV/s.
Pt(S)[n(111)
(110)] stepped surfaces decorated with adatoms [Climent et al., 2001].
Figure 7.12 shows the voltammetric stripping of CO on a Pt(775) stepped surface that
has been modified by selectively depositing different adatoms on step sites. As
expected, no enhancement of CO oxidation is observed for Bi and Te, since oxidation
of these adatoms adsorbed on step sites takes place at potentials much higher than that
of CO oxidation. In these cases, the CO stripping peak is displaced towards positive
values, owing to the blocking effect of the step sites, which are the most reactive
for this reaction [Lebedeva et al., 2002]. However, as mentioned above, As on steps
undergoes a surface oxidation process at 0.57 V, and therefore is capable of providing
the oxygenated species needed for CO oxidation, acting as a bifunctional catalyst. This
is reflected in a significant displacement of the CO oxidation peak towards lower
potential values.
7.6.2 Electrocatalysis of the Poison Formation Reaction
The effect of adatom deposition on the poison formation reaction from formic acid dis-
sociative adsorption has been extensively studied [Climent et al., 1998; Herrero et al.,
1993, 1994, 1995b, c; Llorca et al., 1994]. The poisoning reaction takes place spon-
taneously under open circuit conditions when the electrode is immersed in a solution
containing formic acid. Then, the poisoned surface can be transferred to an electro-
chemical cell containing a solution without formic acid, and the amount of poison
formed can be easily characterized by cyclic voltammetry, through the stripping
charge. In this way, the poisoning reaction can be easily separated from the direct








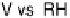

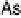













Search WWH ::

Custom Search