Environmental Engineering Reference
In-Depth Information
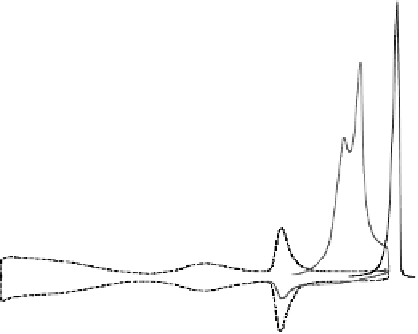
Figure 7.11 CO stripping experiment for a Pt(111) electrode with u
Bi
¼ 0.14 (a) and recovery
of the electrode surface after CO removal (b). Curve (c) shows the CO stripping for the
unmodified surface. Sweep rate: 50 mV/s.
†
At low CO coverages, the adatom oxidation peak can be distinguished from the
CO oxidation peak. Lateral interactions between CO and the adatom stabilize the
elemental Bi state, increasing the potential of the adatom redox peak. For As, a
displacement of the redox peak to lower potentials is observed, indicating an
stabilization of the As(III) state on the CO-As mixed adlayer.
†
For Se and Te, oxidation of the adatom takes place at potentials higher than that
of CO oxidation. The adatom is always in its reduced state, and no bifunctional
catalysis through the transfer of oxygen from the adatom to the CO molecule can
take place.
Fewer studies have been carried out for electrodes with other crystallographic
orientations, since normally the oxidation of the adatom takes place at potentials
higher than that of CO oxidation, and hence no bifunctional enhancement is expected.
For Pt(100), formation and oxidation of mixed CO adlayers with Bi, Te, and Sb ada-
toms have been studied [Feliu et al., 1996]. In all three cases, CO forms mixed
adlayers, although some segregation of the two species was observed at low CO cov-
erages. While for Bi and Te no electrocatalytic effect is observed, for Sb the CO strip-
ping peak potential is reduced by approximately 60 mV. This observation agrees with
the hypothesis that a bifunctional mechanism operates in the electrocatalysis of CO
oxidation on Pt electrodes modified by sp
n
elements, since only adsorbed Sb has an
oxidation potential lower than CO. In this case, a displacement of the Sb redox
peak towards lower potentials due to CO coadsorption is observed, indicating a
destabilization of the elemental Sb state. CO oxidation has also been studied on
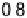
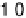

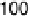






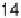

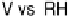

















Search WWH ::

Custom Search