Environmental Engineering Reference
In-Depth Information
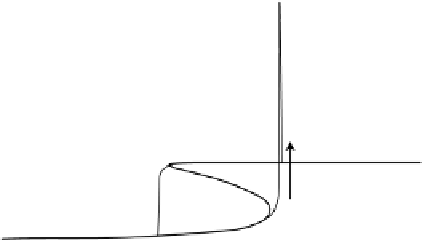
Figure 6.8 S-shaped polarization curve observed in the CO oxidation model (for the exact
model parameters, see Koper et al. [2001]). The thin line shows the cyclic voltammetry observed
at a low scan rate of 2 mV/s.
always higher than the values for the CO stripping peak. This effect is due to the
quenching or self-poisoning effect of the CO incoming molecules in the autocatalytic
process of CO oxidation; i.e., when the oxidation rate is low, the new free sites that are
created in the initial stages of the oxidation are immediately covered by CO molecules
from solution, thereby preventing a fast oxidation of the CO molecules. In order
to achieve a sustained oxidation of the CO molecules, a higher CO oxidation rate
constant is required, which necessarily implies higher potentials. An additional
consequence of this is the dependence of the potential E
2
on the mass transport
rate, as observed in Fig. 6.9 for different rotation rates of the electrode.
Figure 6.9 shows the experimental voltammetry on a Pt(110) electrode in HClO
4
in
a rotating disk configuration. The experimental curves in Fig. 6.9 display all the
qualitative characteristics of the model, and the influence of the scan rate and the
disk rotation rate are well reproduced by the model. In the negative scan and at
fast sweep rates, negative currents have been measured for E , E
1
(see, e.g.,
Fig. 6.9 at 200 mV/s). This negative current is associated with anion displacement
from the surface. At positive potentials, where the stable oxidation current is obtained,
the CO coverage is very low, and anions are adsorbed on the free electrode surface.
When the potential is scanned in the negative direction, the CO coverage
increases abruptly at E ¼ E
1
, where the CO oxidation rate becomes lower than the
supply of the CO molecules transported to the surface. The adsorption of the CO on
the surface displaces the anion from the surface, giving rise to the observed negative
current.
In order to prove the S-shaped character of the polarization curve, the system was
studied galvanostatically. The model predicts that the “sandwiched” branch of the
polarization curve should be stable, and therefore measurable under galvanostatic con-
ditions. Figure 6.10 shows the results of the experiment: depending on the scan rate, an
S-shaped curve can be observed in the back scan, i.e., from high to low current. At low




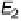



















Search WWH ::

Custom Search