Environmental Engineering Reference
In-Depth Information
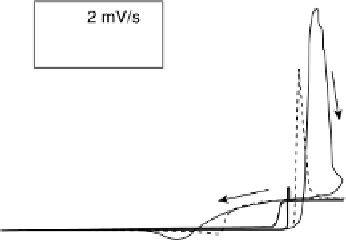
Figure 6.9 Cyclic voltammetry of a Pt(110) rotating disk electrode in a CO-saturated 0.1 M
HClO
4
solution. (a) Influence of the voltage scan rate. (b) Influence of the disk rotation rate.
scan rates, however, the curve is not clearly S-shaped, and irregular potential oscil-
lations are observed. These potential oscillations are most likely due to a spatial
instability, which causes the electrode to exhibit patches of low potential and patches
of high potential simultaneously. The instability would not have the time to fully
manifest when the current is scanned rapidly, explaining why the S-shaped curve is
observed under these conditions. The possibility of such a spatiotemporal instability
in an electrochemical system with an S-shaped polarization curve was proved
mathematically by Mazouz and Krischer [2000]. The same group has also studied
this system experimentally (albeit using polycrystalline Pt), and indeed observed
spatiotemporal phenomena that suggest that the system should not be considered as
homogeneous [Bonnefont et al., 2003].
Similar studies have been carried out with Pt(111) and stepped surfaces with (111)
terraces [Angelucci et al., 2007a, b]. The voltammetric profiles of these surfaces agree
qualitatively with those depicted in Fig. 6.9. For the stepped surfaces, the potentials E
1
and E
2
depend linearly on the step density for terraces wider than 5 atoms. This linear
dependence is a consequence of the dependence of the oxidation rate on the step den-
sity, as was observed in the chronoamperometric CO stripping experiments. In H
2
SO
4





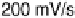
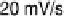
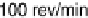





















Search WWH ::

Custom Search