Information Technology Reference
In-Depth Information
the molar extent of reaction
X
is defined by
n
a
a
n
b
b
n
c
c
n
d
d
X
=−
=−
=
=
(10.2)
where
n
i
are the moles of component
i
either created or consumed. The applicable
material balance equations with the feeds and products of component
i
,definedas
F
i
and
P
i
, respectively, are for a reactant. For example, if
i
is component A,
P
i
=
F
i
−
aX
(10.3)
and for a reaction product, for example, if
i
is component D,
P
i
=
F
i
+
dX
(10.4)
This is illustrated at Chapter Ten Examples/RStoicExample and is shown in Figure 10.1.
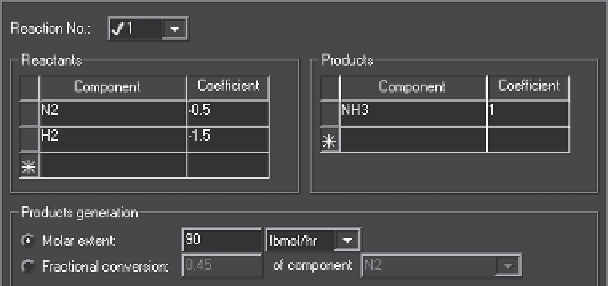
Here a duplicator block feeds each RStoic reactor block. The reaction involved is the
formation of ammonia from hydrogen and nitrogen. The reactor ST1 is configured for
an extent of reaction of 90 lbmol/hr and reactor ST2 with a fraction converted of 0.50
nitrogen. The details of the extent of reaction configuration is shown in Figure 10.2.
11
ST1
21
D1
1
DUPL
12
ST2
22
Figure 10.1
RStoic example.
Figure 10.2
Configuration of extent of reaction.