Chemistry Reference
In-Depth Information
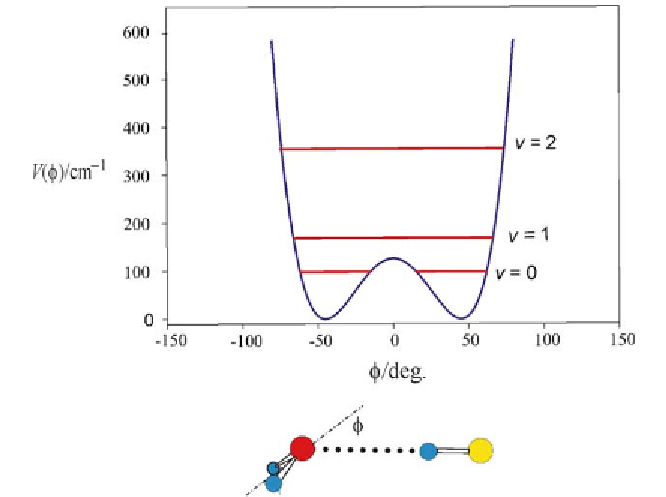
Fig. 2
The experimentally determined potential energy
V
(
φ
), expressed as a wavenumber
for convenience, as a function of the angle
HF.
The definition of
φ
is shown. The first few vibrational energy levels associated with this
motion, which inverts the configuration at the oxygen atom, are drawn. The PE barrier at
the planar conformation (
φ
in the hydrogen-bonded complex H
2
O
···
= 0) is low enough that the zero-point geometry is effectively
planar (i.e. the vibrational wavefunctions have C
2
v
symmetry, even though the equilib-
rium configuration at O is pyramidal with
φ
φ
e
= 46
◦
(see text for discussion)). See Fig. 1
forkeytothecolourcodingofatoms
pression for the conventional quartic/quadratic PE function in terms of the
dimensionless reduced coordinate
z
given in Eq. 1. This function was fitted
to a range of experimental data to give the potential constants
a
and
b
and
then converted to the equivalent
φ
-dependent form of the type given in Eq. 2,
where
is the inversion angle defined in Fig. 2. The form of the reduced mass
for the inversion motion and details of the calculation are given in [112]:
φ
V
(
z
)=
a
(
z
4
-
bz
2
)
(1)
4
-
B
2
V
(
φ
)=
A
φ
φ
(2)
We note from Fig. 2 that the hypothetical equilibrium conformation is pyra-
midal, with
φ
e
= 46(8)
◦
, even though the geometry of the complex is effec-
tively planar in the zero-point state (i.e. the vibrational wavefunction has C
2
v
symmetry) because the PE barrier at the planar (
=0) formis low. At the
time of the publication of [112] this was a critical result because it demon-
φ
Search WWH ::

Custom Search