Chemistry Reference
In-Depth Information
Scheme 8
The two main types of halogen bond interactions
Table 1
Contact distances predicted by the anisotropic model [56] (reproduced from
ref [15], with permission of the ACS)
D
anis
(type I) (A)
D
anis
(type II) A
Interaction
C - Cl
···
Cl
3.56
3.36
C - Br
···
Br
3.68
3.38
C - Br
···
I
3.97
3.67
···
C - I
Cl
3.91
3.54
C - I
···
Br
3.97
3.60
C - I
···
I
4.26
3.89
C - Cl
···
N
-
3.18
C - Br
···
N
-
3.14
C - I
···
N
-
3.36
C - I
···
O
3.67
3.30
C - I
···
S
4.16
3.79
the corresponding
D
anis
value. Note that I
I distances reported above for
E
-TTFPh
2
I
2
(type I, 4.12
A
)and
E
-TTFI
2
(type II, 3.7-3.8
A
) are indeed
shorter than the
D
anis
values for a C - I
···
I interaction reported in Table 1 as
4.26A for a type I and 3.89A for a type II interaction, respectively.
In the numerous examples of
ortho
-dihalo TTFs described so far
(Scheme 7) [53, 57-62], one observes the formation of inversion-centred
dyads with strong S
···
Se interactions (Fig. 4), a recurrent
motif among TTFs with
C
2v
symmetry [63]. This dyadic motif eventually in-
teracts with neighbouring ones through short Hal
···
S, S
···
Se or Se
···
···
Hal contacts and also
Hal
S(Se) contacts, demonstrating that the favoured packing of such TTFs
into dyads with the associated S(Se)
···
···
S(Se) van der Waals interactions over-
comes in most cases possible Hal
Hal interactions. The situation is reversed
in the tetrahalotetrathiafulvalenes [57, 64] where the larger size of the halo-
gen atoms hinders such dyadic association. As a consequence, type I motifs
can now be observed in the chloro and bromo derivatives, while type II motifs
are found in the structure of tetraiodotetrathiafulvalene (Fig. 5) with the fol-
lowing structural characteristics: I
···
I 3.85
A
,
2
= 166
◦
and 3.99
A
,
1
= 87
◦
,
···
θ
θ


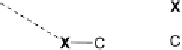
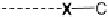
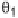
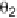
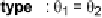
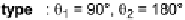










Search WWH ::

Custom Search