Chemistry Reference
In-Depth Information
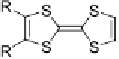
Scheme 6
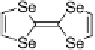
Mono- or
E
-dihalo TTFs
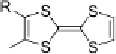
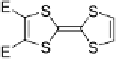
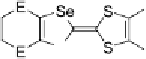
Scheme 7
Examples of
ortho
-diiodo TTFs and selenium analogues used in cation radical
salts
Considering the eventual presence of halogen bonding interactions in the
X-ray crystal structures of these
neutral
molecules, one observes different
behaviours depending on the substitution pattern and number of halogen
atoms on the TTF core. Monohalo TTFs often exhibit a Me/halogen disorder
indicative of the absence of any strong directional halogen bonding inter-
action. Among dihalo TTFs, the
E
-TTFI
2
[54] and
E
-TTFPh
2
I
2
[55] exhibit
intermolecular I
···
I interactions. They are characterized by relatively short
I intermolecular distances (4.12(2)
A
)andatypeImotif(
2
= 120
◦
)
I
···
θ
1
=
θ
in
E
-TTFPh
2
I
2
,whilemuchshorterI
···
I distances with type II motifs (I
···
I
3.676(3)
A
,
and 3.817(3)
A
,
θ
1
= 88.7(6)
◦
,
θ
2
= 175.9(5)
◦
θ
1
= 87.7(5)
◦
,
θ
2
=
175.9(6)
◦
) are found in
E
-TTFI
2
. Note that the evaluation of “short” Hal
Hal
intermolecular distances is based here and in the following on the polar flat-
tening model [56], which attributes to each halide an
r
min
and
r
max
rather
than one single van der Waals radius. In this anisotropic model, the contact
distance (
D
anis
in Table 1) for a type I interaction C - X
···
Y corresponds to
the sum (
r
max
(X) +
r
max
(Y), while the contact distance for a type II interac-
tion corresponds to
r
min
(X) +
r
max
(Y) (Scheme 8). Representative values are
collected in Table 1 for comparison with experimental data. Halogen bond-
ing will be considered as relevant when the X
···
···
Y distance is shorter than

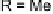
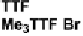
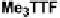

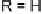
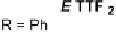


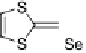
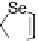
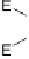

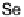
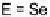



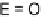
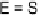
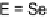
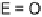
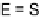
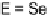



























Search WWH ::

Custom Search