Chemistry Reference
In-Depth Information
Note that this phase diagram points to a very useful fact, namely that when
a hydrogen-bonded (or, indeed, a halogen-bonded) complex has the two com-
ponents in the correct ratio, it will melt as a single entity and there will be
no biphasic behaviour (indeed, it was the lack of such well-defined behaviour
that led to the careful examination of the phthalic acid/stilbazole system in
thefirstplace).However,onecomplicationcanbethepresenceofmultiple
thermal events. For example, complex
11
fails to form from the two compo-
nents after evaporation of the solvent, rather forming an intimate mixture.
On heating, the cyanostilbazole first melts to give a mixture of the crystalline
phase of the acid and the isotropic phase of the stilbazole. At a higher tem-
perature, the complex does, however, form giving rise to a nematic material
that clears in the normal way. Cooling leads to decomplexation and the whole
cycle is repeated [36].
Another important issue relates to the behaviour at the transition to the
isotropic liquid (known as
clearing
) and the question of whether the rup-
ture of the hydrogen bond drives the clearing process or whether the complex
passes from mesophase to isotropic as a complete unit. Simple consider-
ation of complex
10
gives an immediate answer, for these materials clear
around 50
◦
C, at which temperature the benzoic acids are solids. Clearing
should then lead to immediate crystallisation, which is not observed. This
conclusion is reinforced by a variable temperature electronic spectroscopy
study of the behaviour of decyloxystilbazole and 2,4-dinitrophenol [37,
38]. 2,4-Dinitrophenol is a relatively strong acid (p
K
a
= 3.96) and the
study showed that while at room temperature, a neutral hydrogen-bonded
species existed (-N
H-O-), at higher temperature through the SmA
phase, proton transfer occurs to give the ionic hydrogen-bonded species
(-NH
+
···
-
O-) and that this species persists beyond the clearing point. In-
deed, there is no reason why hydrogen bond strength should limit the
stability of a mesophase, for studies of the hydrogen-bonded complex be-
tween the two, non-mesomorphic components, 4-biphenylcarboxylic acid
and 4-cyanostilbazole (
12
), have shown the existence of a nematic phase to
temperatures above 200
◦
C [36].
···
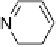

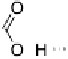
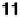


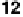






















Search WWH ::

Custom Search