Chemistry Reference
In-Depth Information
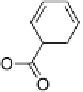
This work raises some interesting issues. The first is that the stoichiom-
etry of a complex is not necessarily the most obvious. For example, it was
reported initially that phthalic acid formed a 2 : 1 complex with alkoxystil-
bazole [34], when in fact a careful study carried out by constructing a binary
phase diagram (Fig. 11) revealed the complex to have a 1 : 1 ratio of the two
components [35]. The reluctance of the system to form the more obvious 2 : 1
complex may relate to the presence of intramolecular hydrogen bonding or
couldevenrelatetothechangeinthep
K
a
of the second acid proton on com-
plexation.
Fig. 11
Binary phase diagram between phthalic acid and decyloxystilbazole. (Cr
c
and Cr
a
are the crystal phase of the complex and the acid, respectively; E is the crystal smectic E
phase). Adapted from [35]

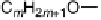











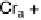
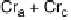
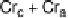

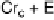
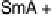
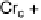
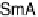
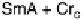

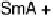

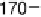

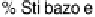
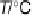
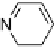
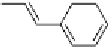
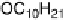






















Search WWH ::

Custom Search