Chemistry Reference
In-Depth Information
electrostatic in nature. The perpendicular arrangement arises primarily from
steric interactions at the
positions.
The reactivity of the initial halogen bonded complex has also received con-
siderable attention. Husebye and coworkers have suggested a process whereby
the dihalogen bond is cleaved to give a key cation intermediate and a halide
anion (Eq. 4) [182]. This mechanism is consistent with that often proposed for
nitrogen electron donor systems.
α
Equation 4
Devillanova and coworkers have also addressed this issue with some sim-
ple thiones and selones. Using spectroscopic analysis and quantum me-
chanical calculations, they examined the various possible reaction pathways
shown in Fig. 5 [72, 183]. The geometries and relative stabilities of the charge-
transfer and “T-shaped” hypervalent adducts were compared using DFT cal-
Fig. 5
Pathways for the reaction of thiones and selones with dihalogens
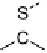



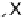
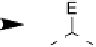
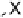
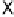


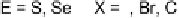



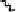
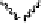
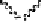

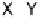

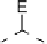
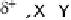
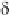
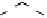
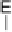
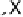
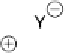
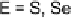

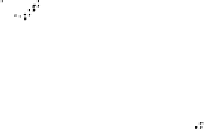










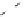
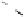






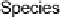
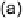
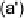
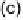
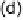
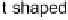
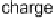

















































































































































































Search WWH ::

Custom Search