Environmental Engineering Reference
In-Depth Information
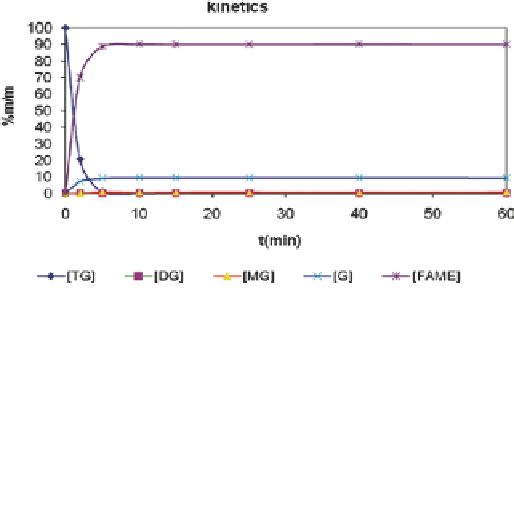
Fig. 2
Concentrations of mono-, di-, triglycerides, FAME and glycerol vs. time in the kinetic
experiments
A + bB
→
k
products
Here the function that expresses the rate of reaction vs. time was
[19]
:
C
C
( )
ktbC M
· ·
·
−=
1
ln
B
, where M =
B0
A
bMC
·
·
bC
.
0
A
A0
The simplified reaction was:
TG + 3CH OH
→
k
G + 3FAME
3
Here C
A
was the concentration of TG, C
B
the concentration of methanol, b = 3. 760
mL (699.2 g) of waste olive oil (mean mw 885.43 g/mol), and 255 mL (201.45 g) of
methanol (a total of 1015 mL) was introduced to the reactor. Thus, the mol number
n
and the initial concentrations C
I0
are: n
A0
= 0.79 mol; C
A0
= 0.78 mol/L; n
B0
= 6.3
mol; C
B0
= 6.20 mol/L. M = 2.65.
Consequently, the second order reaction equation remains as follows:
C
( )
k · t ·3·0.78· 2.65 1
−=
ln
3·2.65·
B
C
A
This second order kinetic equation was solved using the mass fractions of TG and
methanol. The mass fraction of methanol was calculated in every point from the
difference of the measured mass fractions of G, MG, DG, TG and FAME. The
kinetic plot of ln(C
MeOH
/aC
TG
) vs. time gave a straight line (Fig.
3
). The 95% confidence
limits for the intercept and slope of the straight line are:
Slope: 0.2245 ± 0.0521
Intercept: −0.8571 ± 0.1621
The resulting rate constant
k
is 0.2245 L·mol
−1
·min
−1
. The transesterification
reaction of used olive frying oil with methanol catalyzed with sodium methoxide,
follows the equation: