Biomedical Engineering Reference
In-Depth Information
―carrier‖ such as lactose (60-80µm), where the drug should be liberated from
the surface of carrier particles on inhalation (Bell et al., 1971; Dalby et al.,
1996). The extent of drug liberation from the carrier is highly dependent on
formulation and design of inhalation device. DPI devices may be classified
into three types which are: single unit-dose inhalers, multi-unit dose inhalers
and multi-dose reservoir inhalers (Daniher and Zhu, 2008).
In single-unit dose inhalers the drug and carrier are stored in disposable
hard gelatin capsules. The Spinhaler
®
(Rhône-Poulence Rorer) is an example
in which the capsule containing the formulation is fitted onto a rotor and
pierced by two needles. Inhalation by patient induces turbulence within the
device causing the rotor to rotate rapidly with subsequent release of the stored
powder from the capsule through the holes made by the needles.
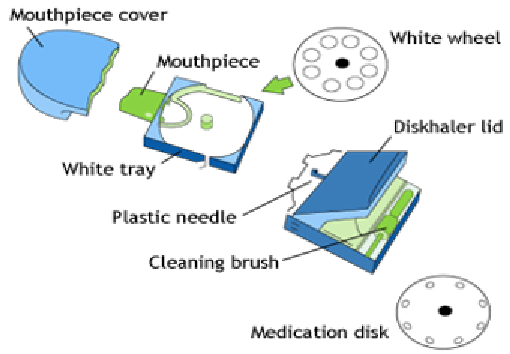
Multi-unit dose inhalers may be represented by the design of the
Diskhaler
®
device in which the drug and carrier particles are filled into foil
blister disc containing multiple doses (Figure 6). The blisters are pierced by
needles present within the device when the lid is raised by the patient. The
powder mix is dispersed into aerosol as patient inhales through the device,
resulting in the delivery from the pierced foil. The Diskhaler is prepared for
the next dose by pulling the disc out and in. Lifting the lid means a new blister
will be pierced for inhalation of the stored medication.
(Source:
www.asthma)
.
Figure 6. A schematic diagram of the Diskhaler DPI device which contains drug doses
within foil blisters in the medication disk. Pulling the lid causes a blister to be pierced
by a plastic needle for patient to inhale the enclosed dose.