Environmental Engineering Reference
In-Depth Information
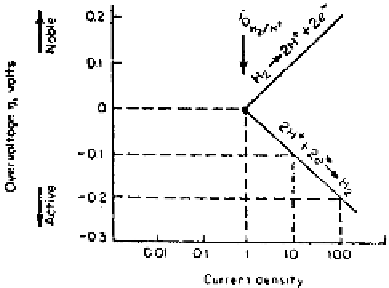
Figure 2.12
Activation-polarization curve of a hydrogen electrode.
Dissolution reactions (anodic) in corrosion are usually controlled by activation
polarization where the solvation of ion is the probable rate-controlling step. Hy-
drogen evolution reactions (cathodic reactions) are controlled by activation polar-
ization where the concentration of hydrogen ions is high.
Concentration Polarization
A buildup or depletion of ions at the electrode surface as a result of reaction will
change the value of the electrode potential according to the Nernst equation. For
example, for a corroding zinc electrode, concentration of zinc will increase with
dissolution in the vicinity of the electrode. The value of
a
oxid
in the equation
increases, causing the electrode potential to shift in a positive direction.
For the hydrogen evolution reaction, the higher rate of discharge of hydrogen
ions at the electrode surface brings down the value of
a
oxid
and the electrode
potential according to the Nernst equation, shifts in a negative direction. How-
ever, the rate of discharge of hydrogen ions at the electrode surface is dependent
on the diffusion of hydrogen ions from the bulk of the solution to the surface;
a maximum or limiting value of this reduction reaction is given by
DnFC
x
i
L
(2.40)
where
i
L
is called limiting diffusion current density, amp/cm
2
D
is the diffusion coefficient for H
ion
n
is the number of electrons transferred
F
is the Faraday number
C
is the bulk concentration of H
ions in the solution and
x
is the thickness
of diffusion layer adjacent to the electrode surface through which the concen-