Environmental Engineering Reference
In-Depth Information
9.3.3.
Oxidized Functional Groups
The partially oxidized organic compounds contain acid, aldehyde, ketone, ether, and
alcohol functional groups. (See Table 9.5.) Each functional group makes up a family of
compounds having different reactivity, solubility, and interaction with various
constituents in the environment. They are generally more soluble in water and move more
readily in the environment than do hydrocarbons. In many cases they are also more easily
decomposed by microorganisms to CO
2
and water.
Organic molecules can contain more than one functional group. Common examples are
sugars (hexoses), which contain five alcohol (-OH) groups, and one aldehyde (-CH=O)
group, as seen in Figure 9.4. Another common example is unsaturated fatty acids, which
contain one acid functional group and one or more double bonds. Amino acids contain at
minimum one acid functional group and one amine group.
Increasing the number of oxygen- or nitrogen-containing functional groups in a
compound increases its solubility. In Figure 9.4 six carbon hexane is very insoluble in
water, while the six carbon hexose sugar is very soluble. Sugars are polymerized into
starch and cellulose, fatty acids polymerize into fats, and amino acids polymerize into
proteins, and when this happens the size of the polymer and its linkages will control
solubility rather than numerous oxygen- or nitrogen-containing functional groups.
When larger organic compounds degrade in the environment, they undergo a series of
decomposition steps during which a number of different intermediate organic compounds
are produced. These often contain a number of different functional groups. The rate of
decomposition of starting and intermediate compounds is related to the functional groups
they contain. Functional groups control a compound's solubility in water. They also
control its rate of decomposition, because generally, the more water-soluble a compound
is the faster it is degraded. The more soluble a compound is, however, the more likely it
is to move in the environment, and thus there will be an increased need for sampling.
This also means that when looking for a particular organic contaminant in soil or water
its natural occurrence must be established and quantified. It cannot be assumed that the
compound of interest is not naturally or commonly present in a particular environment.
This even means that highly reduced compounds such as alkanes, alkenes, and alkynes
may be present even though their formation in aerobic environments is
TABLE 9.5
Organic Functional Groups
Group
Structure
Characteristics
CH
3
(CH
2
)nCH
3
Alkanes
Contains only carbon and hydrogen. Gases and liquids
insoluble in water, flammable.
Alkenes
Contains at least one double bond. Gases and liquids
insoluble in water, flammable.
Alkynes
Contains at least one triple bond. Gases and liquids
insoluble in water, flammable.

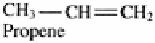
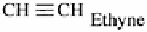

Search WWH ::

Custom Search