Environmental Engineering Reference
In-Depth Information
Both H
+
and act as cations in soil and become exchangeable cations on the
cation exchange capacity. Although protons H
+
, or H
3
O
+
are cations, it is hard to define
their exact cation exchange activity. Ammonium however, acts like any other
cation in the soil and can readily be identified as an exchangeable cation. Both these
cations can affect cation availability and exchangeability in soil analysis. Exposure of
samples to either changes in pH or ammonia thus can change analytical results.
9.2.5.
Cation Exchange Capacity
Clay and organic matter in soil usually have a negative charge and thus attract and hold
cations. This results in the soil having cation exchange capacity (CEC). It should be
expected that a portion of all cations in soil will be associated with the cation exchange
sites. This includes both metal and nonmetal cations.
Analytical methods cannot directly measure the amount of cations on the exchange
sites; they must be removed from the sites and brought into solution before analysis. This
means that some replacing cation must be used to exchange with them and move them
into solution. A cation that has higher oxidation states (2
+
and 3
+
) or that is present at
high concentration will replace cations with lower oxidation states or lower
concentrations.
Some cations are not attracted to CEC sites in soil. Most iron (Fe
3+
), aluminum,
titanium, and manganese precipitate as oxides and thus are not available to act as soluble
or exchangeable cations. On the other hand, Fe
2+
forms soluble complexes with organic
compounds, and thus it also does not act as a simple cation. It should be expected,
however, that both oxidation states of iron will be found in most soils.
Although cation exchange capacity is most often discussed, some soils also show
appreciable anion exchange capacity. Some tropical and highly weathered soils are
particularly noted for this characteristic. Just as in cation exchange, an anion must be
removed from anion exchange sites to be analyzed. It is also important to remember that
not all anions (e.g., phosphate) are either excluded or attracted to soil particles in an
exchangeable fashion [16].
9.2.6.
Anions
Anions in soil are single oxidation state species, such as the halogens and the
multioxidation state oxyanions. Only the halogens are commonly found in the
environment as simple anions and are found with an oxidation state of negative one.
Because most soil particles and the simple anions have negative charges they repel each
other. Thus the simple anions are readily leached lower in the soil or out of it altogether
[17]. The oxy anions may or may not be readily leached out of soil, depending on their
ability to chemical react with other ions or species.
Many oxyanions occur naturally in the environment. The most important of these are
carbonate, nitrate, phosphate, and sulfate. There are many others, however, that are in
lower concentration but are nevertheless extremely important. Important oxyanions, their
structures, and their biological importance are given in Table 9.2.

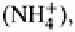
Search WWH ::

Custom Search