Environmental Engineering Reference
In-Depth Information
9.2.6.1. Carbonate and Bicarbonate
In addition to their biological importance, carbonate and bicarbonate are important in
determining the buffering and pH of soil and soil solutions. Carbon dioxide from
respiration in plant roots and microorganisms is released into the soil atmosphere. Some
dissolves in water, forming bicarbonate ions.
If the existing soil solution becomes acidic, bicarbonate will accept a proton-
TABLE 9.2
Common Environmental Oxyanions
Name
Formula
Source and biological importance
Carbonate
Counter and exchange ion in root uptake of cations and anions
Bicarbonate
Counter and exchange ion in root uptake of cations and anions
Sulfate
Source of sulfur for plants
Nitrate
Source of nitrogen for plants
Nitrite
Source of nitrate in soil
Phosphate
Source of phosphorus for plants
Molybdenate
Source of molybdenum for plants and microorganisms; important in
nitrogen fixation
Arsenate
Toxic component in the environment
Selenate
Source of selenium for plants and animals
Borate
Source of boron for plants
producing carbon dioxide and water, the reverse of the equation above. The common test
for carbonate minerals is
C
aCO
3
+2H
+
2Cl
−
→
CO
2
+Ca
2+
+2Cl
−
+H
2
O
to treat them with dilute hydrochloric acid. The release of gas in the form of bubbles is a
positive test for carbonate. Soil carbonates form in soil by the following reaction:
2
H
2
O+CO
2
+Ca
2+
→
CaCO
3
↓
+2H
+
In basic environments protons are released from bicarbonate and carbonate is formed as
shown above. If this takes place where there is an excess of calcium or some other base



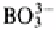



Search WWH ::

Custom Search