Environmental Engineering Reference
In-Depth Information
In this equation the components are assumed to be at equilibrium. The total amount of the
component can be found by summing the amounts in the solid, liquid, and gaseous
phases.
Although over short periods of time the environment may appear to be in equilibrium, it
is never truly in equilibrium. Such equations may provide important information about a
component in the environment, however. They may also be included in more
comprehensive models of the environment [3].
Mass transfer is the movement of a mass of material through a medium. The medium
could be air, water, or soil. There are simple chemical equations for mass transfer;
however, in soil the situation is complicated because there is a limited amount of space
available for transfer. For this reason a capacity coefficient is needed. In soil some
volume containing solvent and solute is immobile and so does not contribute to mass
transfer. To complicate equations and models is the fact that solute can be sorbed to pore
surfaces. If the process is started with dry soil, some solute (water) will be sorbed to the
surface so tightly that it will be immobile. This amount of solute is also part of the
equation.
Two components of mass transfer are spherical diffusion and advective (horizontal)
dispersion. In spherical diffusion a point source of material will tend to diffuse outward
from that sphere in all directions equally. Conceptually there may be a number of spheres
and material diffusing out of them. Another possibility, advective dispersion, is
horizontal movement away from the source. This would be the case in a stratified
medium, in which a denser layer underlies a less dense horizon.
The combined soil-atmosphere model for evaluating the rate of loss of surface-applied
pesticides developed by Rivka Reichman, Rony Wallach, and Yitzhak Mahrer is an
example of this type of model and this type of modeling [4]. This model is specifically
designed to be used with the pesticides lindane, dieldrin, and trifluralin. It is developed
using thermo-chemical-type mass balance equations for water and contaminant in the
atmosphere and soil. Partial differential equations are used for heat, moisture, and
chemical transport. Other equations are used for surface and boundary conditions
between soil and the atmosphere. Once developed, the model can be run using a
computer after input of the appropriate data. The model is then compared to results from
an experimental study.
There is a rather large number of inputs used in most models, including the one above.
In this particular case the model involves 73 constants, variables, and assumed
conditions. In some cases once all the variables are known the answer is also known.
An example of a chemical kinetic model is a modification of the environmental model
called GLEAMS (groundwater loading effects of agricultural management systems) [5]
to simulate mercury transport. This model is called Hg-GLEAMS. Kinetics are used in
this model to represent the transformation of mercury between its various states. These
equations are used to measure the rate of conversion of mercury between Hg
0
, metallic
mercury, and Hg
2+
. Equations take the form of standard chemical rate equations. This

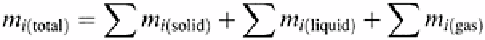
Search WWH ::

Custom Search