Environmental Engineering Reference
In-Depth Information
or regolith profile. This in turn will be important in designing a sampling plan.
Also, the data obtained from evapotranspiration studies will be needed for some
mathematical models to be discussed below [1].
7.2.
CHEMICAL MODELS
Chemical-type models of the environment usually rest or depend on some underlying
chemical principle. Kinetics, equilibrium, solubility, mass transfer, and partition between
organic and aqueous phases have all been used in modeling compounds in the
environment. These approaches work best with single chemical species or two or more
similar species that do not interact with each other. Many mathematical models are also
based on chemical phenomena.
Thermodynamics is the study of energy (heat), its movement, and its effect on its
surroundings. All systems generally move from conditions of higher energy to conditions
of lower energy. They also move from conditions of higher organization to areas of lower
organization. In thermodynamic terms this would be moving to conditions of greater
randomness. In the environment water moves downhill. Water also moves down through
soil and is held more strongly in small pores and less strongly in large pores. To move it
from small to large pores requires the input of energy. Organic matter in aerobic soil is
oxidized to carbon dioxide and water. In anaerobic soil organic matter is also broken
down; however, in this case the end product is methane. In both these cases energy is
given off and the compounds produced are smaller and more disorganized [2]. If a
reaction results in energy being given off and randomness increasing, then it can be
expected to take place.
Looking at the large scale, the soil is a medium designed to break down organic
compounds. It provides an intimate mix of solid surface, inorganic nutrients, water,
oxygen, and living organisms, and all of this is in the correct proportions to promote
rapid decomposition with the consequential release of energy and increase in
randomness. Using all of the above the fate of a component in the environment can be
modeled by knowing and following changes in its energy. The component will move
from situations of higher energy and more organization to situations of lower energy and
less organization.
Equilibrium is also used to develop models of the fate of components in the
environment. The concept of equilibrium is that at a macro scale a condition is reached at
which the concentrations of components on either side of a chemical equation do not
change. This situation is most often a dynamic equilibrium, however, in which reactants
and products are moving back and forth between these two conditions. A standard
representation of a chemical equilibrium reaction is shown and is said to be at
equilibrium when the rate k
1
is balanced by the rate k
−1
.
Equilibrium models such as the one given below are fairly simple and easy to calculate.

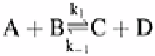
Search WWH ::

Custom Search