Biology Reference
In-Depth Information
Fig. 2.6
Chemical modification pattern of an anti-
Apob
DsiRNA. Sequence of a modified DsiRNA
targeting the
Apob
gene (NM_009693) is shown [
63
]. RNA bases are
uppercase
and 2¢ -OMe RNA
bases are
underlined
; “
asterisk
” indicates a PS (phosphorothioate) internucleotide linkage. Vitamin
E (a-tocopherol) was attached to the 5¢-end of the antisense strand via a phosphate linkage. The
sites of Dicer cleavage are indicated
vitamin E group is a lipid-soluble antioxidant vitamin that can gain entry to all
mammalian cells. Similar to cholesterol, vitamin E associates with serum lipopro-
teins; the SCARB1 scavenger receptor and LDL receptor are involved in cellular
uptake [
64
]. By attaching vitamin E to the 5¢-end of the antisense strand of a
DsiRNA, the ligand became part of the disposable “end domain” of the DsiRNA,
which is cleaved off of the RNA duplex and does not remain attached to the mature
siRNA and, therefore, poses no risk to interfere with RISC entry.
Apob
mRNA lev-
els were reduced by 80% at a dose of 32 mg/kg using this approach.
Kubo and colleagues studied modification of the ends of DsiRNAs with an ali-
phatic amino modifier and found that addition of this simple group could improve
stability and potency of an otherwise unmodified duplex [
65
] . In particular,
modification at the 3¢-end of the sense strand had favorable effects. Consistent with
the earlier finding reported by Kim [
30
], placement of a single modifying group at
the 3¢-end of the antisense strand impaired Dicer processing, while modification of
both of the 3¢-ends prevented Dicer cleavage. This group also conjugated palmitic
acid (C16) at the 3¢-end of the sense strand, a location that permits removal of the
ligand so the modifier is not present in the mature 21-nt siRNA following Dicer
processing (see Fig.
2.5
). The 3 ¢-palmitic acid modification led to additional stabi-
lization of the duplex, and an otherwise unmodified DsiRNA survived 48-h incuba-
tion in 10% fetal calf serum. Addition of the C16 aliphatic chain also promoted
naked delivery of the modified DsiRNA to HeLa cells in tissue culture when used at
a relatively high dose (200 nM).
2.2.2.2
Chemical Modi fi cation and Immune Stimulation
The mammalian innate immune system employs a fixed repertoire of receptors
which recognize structures that are usually associated with pathogens (pathogen-
associated molecular patterns or PAMPs), such as bacterial flagella or bacterial
lipopolysaccharide. The innate immune system is also capable of recognizing
nucleic acids, including single-stranded and double-stranded RNA (ssRNA,
dsRNA); this system probably evolved as a fast-response pathway to viral infection.
Several members of the Toll-like receptor family (TLRs) recognize RNA, such as
TLR3 which binds dsRNA and TLR7 and TLR8 which bind ssRNA. TLR3, 7, and
8 primarily reside in endosomal compartments which limits their contact with
endogenous RNAs, helping to limit the risk of an autoimmune response. Further,
these receptors preferentially recognize unmodified RNA. RNAs bearing several
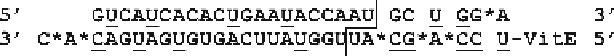
Search WWH ::

Custom Search