Biology Reference
In-Depth Information
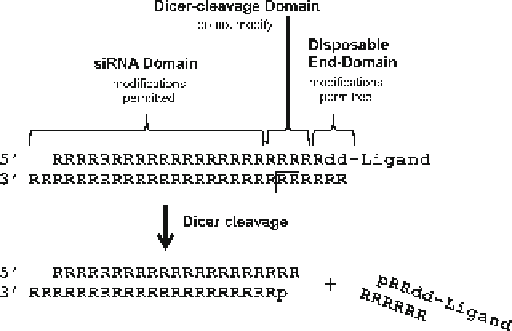
Fig. 2.5
Schematic of DsiRNA domains for chemical modification. An asymmetric 25/27-nt
DsiRNA is shown on the
top line
with the domains suitable for chemical modification indicated by
brackets
. A small interior domain offset to the
right of center
is
not bracketed
, which is the site of
Dicer cleavage; this region should remain unmodified. The preferred attachment site of ligands is
shown on the
right
, connecting to the 3¢-end of the passenger strand. The 21-nt siRNA that results
from Dicer processing is shown along with the discarded cleavage fragment. RNA bases are
upper-
case
and DNA bases are
lowercase
; “p” indicates a 5¢ -phosphate
sponding to the STAT1 site 2 shown in Fig.
2.1
). Like 21-nt siRNAs, some of the
more heavily modified duplexes showed significant impairment of functional
potency, while less highly modified patterns remained potent. 2¢ -OMe modi fi cation
of the sense and/or antisense strands in the “siRNA Domain” (Fig.
2.5
) in an alter-
nating pattern was particularly effective, and this approach to modification was also
shown to work well at additional sites in the human
HPRT1
, mouse
F3
, and
EGFP
genes. The mass spectrometry dicing assay was used to examine processing of a set
of anti-
HPRT1-
modified DsiRNAs, and as long as the modifications did not extend
into the Dicer-cleavage domain, the expected siRNA products were made following
in vitro dicing. Further, DsiRNAs modified with only 11 2¢-OMe residues on the
antisense strand showed a significant improvement in stability when incubated in
serum compared to unmodified 21-nt siRNAs or 25/27-nt DsiRNAs. While this
simple modification pattern is often effective, it can impair potency in a sequence-
specific fashion at some sites, so additional optimization of the precise placement of
modified bases can be beneficial.
Nishina and colleagues described use of a modified asymmetric 27/29-nt DsiRNA
to suppress
Apob
expression in mouse liver [
63
] . 2 ¢-OMe RNA residues and PS
bonds were placed at optimized locations in the sense and antisense strands, avoid-
ing modification of the Dicer cleavage domain. Vitamin E (a -tocopherol) was
attached to the 5¢-end of the antisense strand via a phosphate linkage. The sequence
and modification pattern of this compound are shown in Fig.
2.6
. The modi fi cation
pattern employed six 2¢-OMe residues and a single PS bond in the sense strand and
nine 2¢-OMe residues with five PS bonds in the antisense strand and achieved
sufficient stability to be used via direct naked intravenous injection in mice. The
Search WWH ::

Custom Search