Civil Engineering Reference
In-Depth Information
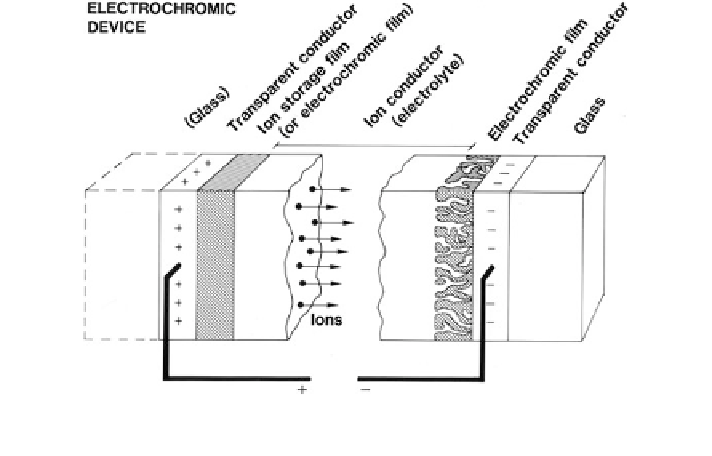
Fig. 2 Basic design of a ''battery-type'' electrochromic device. Arrows indicate ion transport in
an electrical field. From Granqvist (
1995
)
that suitable materials are used, short-circuiting brings back the original properties.
The charging can be interrupted at any intermediate level, implying that the device
has open-circuit memory. Hence, electrical power is needed only to change the
optical properties, not to maintain them, which is important with regard to energy
efficiency. The voltage needed in the electrochromic device is only a few volt DC,
which makes solar cell powering convenient (Lampert
2003
).
The discussion above makes it clear that the electrochromic device can be
described as an electrical battery with a charging state corresponding to a degree of
optical absorption. This analog goes a long way, and the two types of devices share
numerous properties and idiosyncrasies. One example is that they can be damaged
by overcharging or overheating, but that they also exhibit poorly understood ''self-
healing'' features. Another important similarity is that the properties cannot be
changed abruptly, and the coloring/bleaching (or charging/discharging) times may
amount to seconds for a device that is few square centimeters in size, while it can
be minutes or even tens of minutes when the size is of the scale of square meters.
The ''battery-type'' electrochromic device in Fig.
2
works best if it incorporates
two electrochromic films: one coloring under ion insertion and called ''cathodic''
and another coloring under ion extraction and called ''anodic.'' The most well-
known and widely studied cathodic oxides are based on W, Mo, and Nb, whereas
the corresponding anodic oxides are based on Ir and Ni (Granqvist
1995
). Devices
using W oxide together with Ni oxide combine a number of advantageous prop-
erties (Avendaño et al.
2006
; Niklasson and Granqvist
2007
) and are the basis for
several practical electrochromic devices, as we return to in
Sect. 3
. Ir oxide has
excellent electrochromic properties, but it is very rare and precious and hence ill-
suited
for
large-scale
applications;
however,
the
properties
remain
rather