Agriculture Reference
In-Depth Information
b
= 1
k
s
= 0 hr
-1
0.05
0.1
0.25
1.0
0.8
0.6
0.4
0.2
0
0
1
2
3
4
V/V
o
5
6
7
8
9
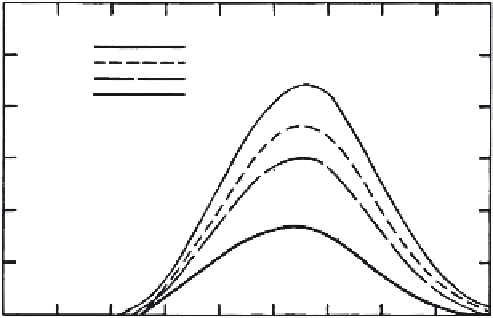
FIGURE 3.20
Breakthrough curves for several values of the rate coefficient
k
5
, where
b
= 1. (From Selim and
Amacher, 1997. With permission.)
rise in concentration or a steep sorption side with an increase of the tailing of
the desorption side for decreasing values of
b
. In contrast, for
b
> 1 the sorp-
tion side indicates a slow increase of the associated concentration and there
is a lack of tailing of the desorption side of the BTCs.
The significance of the rate coefficients
k
1
and
k
2
associated with a kinetic
reaction on solute retention and transport may be illustrated by the BTCs
shown in Figures 3.18 and 3.19 where a range of rate coefficients differing by
three orders of magnitude were chosen. For the BTCs shown in Figure 3.18,
the forward rate coefficients were constant (
k
1
= 0.10 h
-1
), whereas
k
2
varied
from 1 to 0.001 h
-1
. A decrease in concentration maxima and a shift of the
BTCs resulted as the value for
k
2
decreased. Such a shift of the BTCs signi-
fies an increase in solute retention due to the influence of the kinetic mecha-
nism associated with
S
1
. As the rate of backward reaction (
k
2
) decreases
or
k
1
/
k
2
increases, the amount of
S
1
retained increases and solute mobility
in the soil becomes more retarded. The BTCs shown in Figure 3.19 illustrate
the significance of the magnitude of the kinetic rate reactions
k
1
and
k
2
while
the ratio
k
1
/
k
2
remained constant. It is obvious that, as the magnitude of
the rate coefficients increased, the amount of solute retained increased and
an increased solute retardation becomes evident. Moreover, for extremely
small
k
1
and
k
2
values (e.g., of 0.001 h
-1
), the BTC resembles that for a non-
reactive solute due to limited contact time for solute retention by the soil
matrix under the prevailing water-flow velocity conditions. On the other
hand, large rate coefficients are indications of fast or instantaneous reten-
tion reactions. Specifically, rapid reactions indicate that the retention pro-
cess is less kinetic and approaches equilibrium conditions in a relatively
short contact time.
Search WWH ::

Custom Search