Agriculture Reference
In-Depth Information
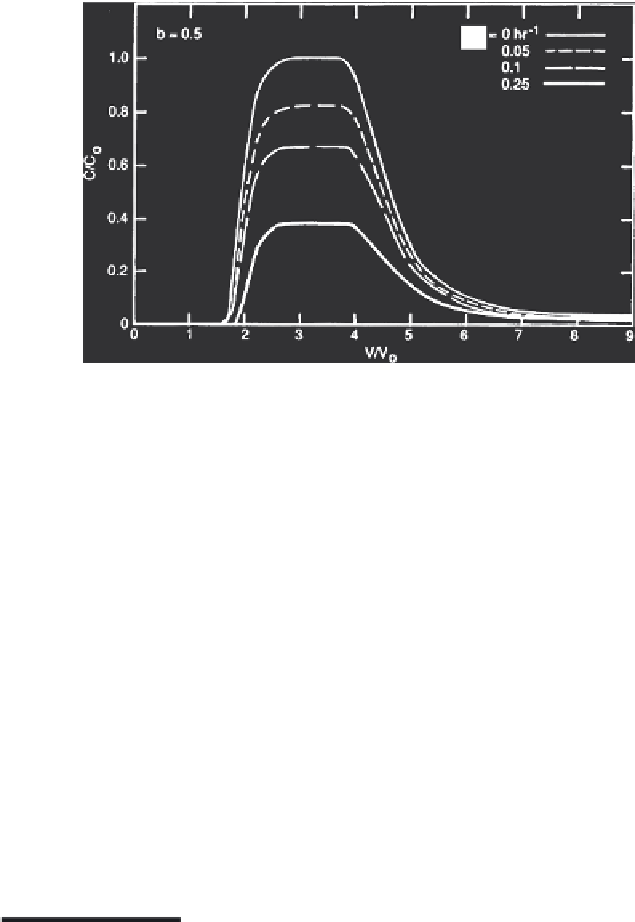
b
k
s
FIGURE 3.21
Breakthrough curves for several values of the rate coefficient
k
5
, where
b
= 1. (From Selim and
Amacher, 1997. With permission.)
In the BTCs shown in Figure 3.19, the irreversible retention mechanism for
solute removal via the sink term was ignored (i.e.,
Q
= 0). The influence of the
irreversible kinetic reaction is straightforward, as shown in Figures 3.20 and
3.21. This is manifested by the lowering of the solute concentration for the
overall BTC for increasing values of
k
s
. Since a first-order irreversible reaction
was assumed for the sink term, the amount of irreversibly retained solute
and thus lowering of the BTC is proportional to the solution concentration.
The primary difference between the BTCs shown in Figures 3.20 and 3.21 is
due to the value of the nonlinear parameter
b
associated with the Freundlich
equilibrium retention mechanism. In Figure 3.20,
b
= 1, whereas
b
= 0.5 for the
simulations shown in Figure 3.21. All other parameters remained invariant
(
k
1
= 0.001,
k
2
= 0.01 h
-1
, and
K
f
= 1 cm
3
/g ).
References
Andreini, M. S., and T. S. Steenhuis. 1990. Preferential Paths of Flow under
Conventional and Conservation Tillage.
Geoderma
. 46: 85-102.
Bear, J. 1972.
Dynamics of Fluids in Porous Media
. New York: Elsevier.
Bond, W. J. 1986. Velocity-Dependent Hydrodynamic Dispersion During Unsteady,
Unsaturated Soil Water Flow: Experiments.
Water Resour. Res
. 22: 1881-1889.
Bond, W. J. and D. E. Smiles. 1983. Influence of Velocity on Hydrodynamic Dispersion
during Unsteady Soil Water Flow.
Soil Sci. Soc. Am. J
. 47: 438-441.

Search WWH ::

Custom Search