Chemistry Reference
In-Depth Information
3.4.3 uhPlc
In
m
ethod
d
eveloPment
S
yStemS
One of the primary drivers for the growth and continued use of HPLC has been the
evolution of packing materials used to affect the separation. The underlying prin-
ciples of this evolution are governed by the van Deeter equation:
H = A(d
p
) + B/u + C(d
p
)
2
u
which is a formula that describes the relationship among H, plate height (HETP or
column efficiency); linear velocity, u, (flow rate); and particle size or diameter, d
p
.
The “A” term represents eddy diffusion, the “B” term represents longitudinal diffu-
sion, and the “C” term represents resistance to mass transfer in and out of the particle.
According to the van Deeter equation, as the particle size decreases to less than
2 µm, not only is there a significant gain in efficiency, but the efficiency does not
diminish at increased flow rates or linear velocities [15]. By using smaller particles,
speed and peak capacity (number of peaks resolved per unit time) can be extended
to new limits; this has come to be known as Ultra High Pressure LC (UHPLC).
UHPLC takes full advantage of chromatographic principles to run separations using
columns packed with smaller particles, and/or higher flow rates for increased speed,
with superior resolution and sensitivity [16-18].
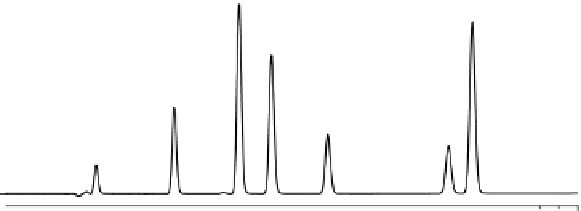
An example of the use of UHPLC for rapid method development is illustrated in
Figure 3.8. The method development process for this rather complex separation was
accomplished in twenty-two preliminary runs, including organic composition scout-
ing, and individual injections for peak identification. Due to the short UHPLC run
times, the entire method was developed in less than an hour. Attempting to do the
same via HPLC could take days to weeks longer, and in the end, HPLC may not be
able to accomplish this result due to its inherent lower efficiency and resolving power.
3
0.28
7
4
0.21
0.14
2
5
6
0.07
1
0.00
0.15
0.30
0.45
0.60
0.75
0.90
1.05
1.20
1.35
1.50
Minutes
FIgure 3.8
UHPLC separation of coumarin and related compounds illustrating fast
method development. Final conditions included a 2.1 by 50 mm 1.7-µm ACQUITY UPLC
BEH C
18
(Waters Corporation, Milford, Massachusetts) column at 35°C. A 5-80% B lin-
ear gradient over 1.0 minute, at a flow rate of 1.0 mL/min was used. Mobile phase A was
0.1% formic acid, and B was acetonitrile. UV detection at 254 nm and 40 pts/s. Peaks
are (1) 7-hydroxycoumarin-gluconoride, (2) 7-hydroxycoumarin, (3) 4-hydroxycoumarin,
(4) coumarin, (5) 7-methoxycoumarin, (6) 7-ethoxycoumarin, (7) 4-ethoxycoumarin.