Chemistry Reference
In-Depth Information
Owner's Site
Vendor's
Site
Functional testing/verification
Owner's Site
Calibration
and
maintenance
Qualification
Structurally
validated
products
Installation
Operational
Performance
DQ
IQ
OQ
PQ
PQ
Prior to
purchase
of a new
type of
instrument
At
installation
(new, old, or
existing
unqualified
instrument)
After
installation
or major
repair of
each
instrument
Periodically
at specified
intervals for
each
instrument
After use
Before
Purchase
Before Use
System Suitability During Use
After Use
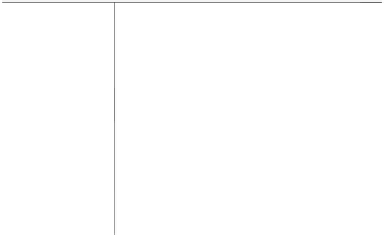
FIgure 2.1
A timeline approach to AIQ.
2.3.3 o
PerAtIonAl
Q
uAlIfIcAtIon
(oQ)
Once the IQ phase is completed, testing is done to verify that the instrument or
instrument modules operate as intended in an OQ phase, as illustrated in Figure 2.1.
First, fixed parameters, for example, length, weight, height, voltage inputs, pressures,
etc., are either verified or measured against vendor-supplied specifications. Since
these parameters do not change over the lifetime of the instrument, they are usually
measured just once. Next, secure data handling is verified. Finally, instrument func-
tion tests are undertaken to verify that the instrument (or instrument modules) meets
vendor and user specifications.
Instrument function tests should measure important instrument parameters
according to the instrument's intended use and environment. In HPLC, the follow-
ing types of tests might be included
• Pump low rate accuracy
• Gradient accuracy
• Injector accuracy
• Column oven and auto sampler temperature
• Detector wavelength accuracy and linearity
• Detector linearity
The analyst would first verify that all the individual modules in the system per-
formed the start-up diagnostic routines successfully, and then each module is tested
individually against predetermined specifications. Relevant OQ tests for each of the