Information Technology Reference
In-Depth Information
fasted and fed states, for both IR and XR formulations, were caused by
the difference in
in vivo
solubility under fasted and fed states.
Another example of using computer simulations to establish IVIVC
referred to etoricoxib solid oral dosage forms (Okumu et al., 2008).
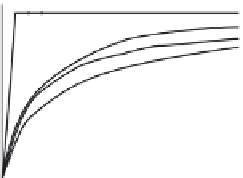
Dissolution profi les of etoricoxib from the fi lm-coated tablets were
performed in USP Apparatus 2 at 75 rpm, using conventional dissolution
media: simulated gastric fl uid (SGF) and USP-simulated intestinal fl uid
(USP-SIF) (900 mL), and fasted state simulated intestinal fl uid (FaSSIF)
(500 and 900 mL) as 'biorelevant' media. The
in vitro
data obtained were
then used as input functions in GastroPlus™ to predict the corresponding
drug absorption profi les (Figure 6.18). A comparison of the simulated
profi les with the
in vivo
observed data (Table 6.13) indicated that the
profi les obtained in SGF and 900 mL FaSSIF appeared to simulate the
in
vivo
profi le better when compared with that in SIF and 500 mL FaSSIF.
These results suggested that USP-SIF might not be the best choice of
media, and that recommended 500 mL FaSSIF (Galia et al., 1998;
Marques, 2004) may not be the right choice of volume for 'biorelevant'
in vitro
testing of etoricoxib tablets. However, the simulation results
based on the dissolution data obtained in 900 mL FaSSIF and SGF
provided a comparatively good IVIVC (r
2
= 0.899 and 0.898, respectively).
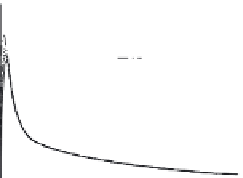
Etoricoxib: (a) comparison of dissolution profi les in the
USP Apparatus 2 (n = 3); (b) comparison of simulated
profi les and observed
in vivo
data (60 mg tablet) using
dissolution data as input function in GastroPlus. The
simulated curves of 0.01 M HCl and 900 mL FaSSIF
are super-imposable and predict the observed data
well; however, the simulated curves using SIF or
500 mL FaSSIF as input function show lower C
max
values (reprinted from Okumu et al., 2009; with
permission of Elsevier)
Figure 6.18




























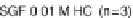










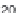




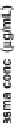


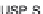

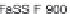



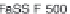















































































































































Search WWH ::

Custom Search