Information Technology Reference
In-Depth Information
IVIVC plot for GLK IR tablets: (a) convolution approach;
(b) deconvolution approach
Figure 6.13
In the attempt to establish IVIVC for the same data set using the
deconvolution approach, the hypothetical GLK
in vivo
absorption profi le
estimated by GastroPlus™ was compared with previously described
in
vitro
dissolution profi les. Since
in vitro
drug dissolution was faster than
the corresponding
in vivo
process, it was necessary to rescale the time
axis when progressing from
in vitro
to
in vivo
. The IVIVC plot of the
percentage dissolved
in vitro
vs. the percentage absorbed
in vivo
, is
presented in Figure 6.13b. The outcomes of deconvolution revealed that
the
in vitro
profi le e (stretched by 12-fold linear rescaling of the time axis)
has the same general shape (morphology) as the estimated hypothetical
in vivo
dissolution profi le, although a good correlation was also achieved
for the
in vitro
profi les b, c, and d (Table 6.10). These results were in
accordance with those obtained by the convolution approach. Since both
convolution and deconvolution approaches were successful in establishing
a level A IVIVC, it was suggested that dissolution specifi cation of more
than 85% GLK dose dissolved in 60 min may be considered as biorelevant
dissolution acceptance criteria for GLK IR tablets.
Other examples can also serve as a good illustration of how GIST can
be used to develop IVIVC. In our previous work (Kovacevic et al., 2009),
a convolution based approach was applied to simulate CBZ plasma
concentration-time profi les based on different
in vitro
dissolution rates,
with the aim to evaluate whether IVIVC for IR and CR CBZ tablets could
be established. Dissolution studies of the investigated IR and CR CBZ
tablets were performed in the United States Pharmacopoeia (USP) rotating
paddle apparatus at 75 rpm, using 900 mL of various dissolution media.







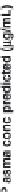















































































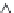




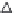






























































Search WWH ::

Custom Search