Civil Engineering Reference
In-Depth Information
distort the lattice surrounding the dislocation, (2) move the dislocation past the lat-
tice defect, and (3) break and reform bonds within the lattice [
21
].
If dislocation motion is not continued, the metal will fracture. In this case, frac-
ture is a result of too many dislocation pileups. As the metal deforms, more dislo-
cations are created and there is a higher dislocation density within the metal. As
the density increases, the separation distance between the dislocations is reduced
and the repulsive forces between the dislocations will increase as the dislocation
density increases. To this end, if a metal is being deformed and dislocations can-
not move, more dislocations are being generated and are being moved until they
come across a stuck dislocation, or a pileup. The addition of dislocations contin-
ues until the repulsive forces between dislocations become too high and cause the
metal to break. One possible way to eliminate dislocations from a metal before
fracture would be to perform an annealing procedure, where the metal is heated to
a material-specific time and temperature setting, and a new lattice structure would
be created from recrystallization with a lower dislocation density.
1.4.3 Crystalline Structures
A crystalline material can be any material that has an actual pattern in which
atoms are situated over a given atomic level distance. The pattern of atoms is the
lattice. There are three main crystal structures for metals, and the separating char-
acteristics of these structures involve spacing, packing, and stacking patterns. For
each crystalline structure, several key characteristics will be discussed, including
the coordination number, the atomic packing factor (APF), and the number of slip
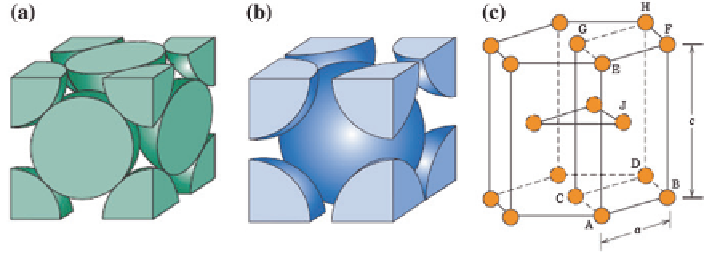
systems. Figure
1.9
shows the unit cells of the three primary crystalline structures.
The coordination number describes the number of neighboring atoms that a single
atom touches within a unit cell of the metal. This can be important for character-
istics such as heat transfer, since thermal conductivity would typically be higher
in metals where more atoms are contacting each other. The APF shows the ratio
of the volume of the atoms to the total volume of a unit cell. This can describe the
Fig. 1.9
Unit cells of the primary crystalline structures [
21
].
a
FCC,
b
BCC, and
c
HCP