Biology Reference
In-Depth Information
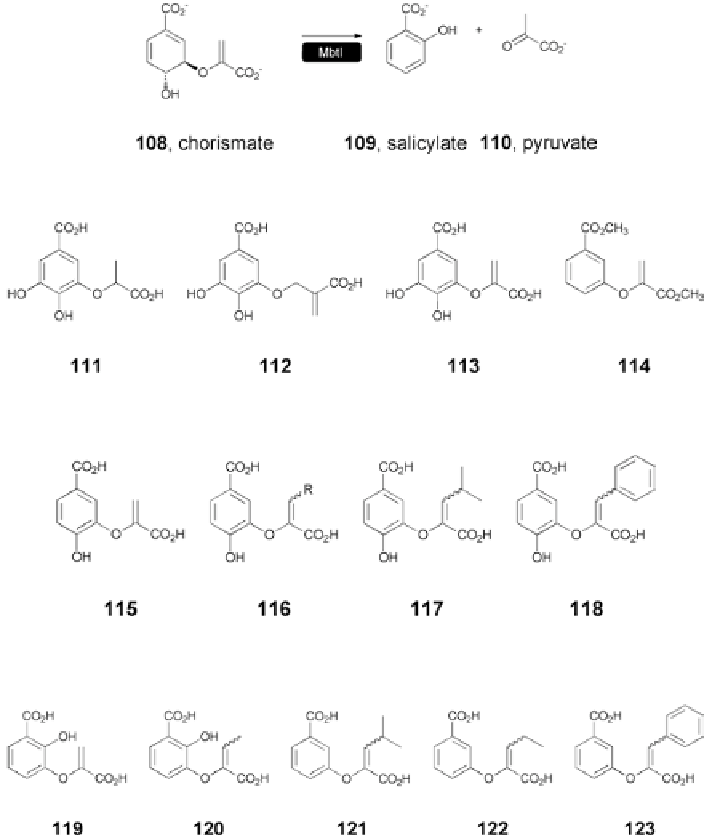
Fig. 5.22
MbtI-catalyzed synthesis of salicylate from chorismate [
80
]
Fig. 5.23
Chorismate-based MbtI inhibitors [
81
]
Fig. 5.24
Chorismate-based MbtI inhibitors. SAR of the C3 position [
81
]
Fig. 5.25
Isochorismate-based MbtI inhibitors. SAR of the C3 position [
81
]
Substitution at the C3 position was also studied, while preserving the hydroxyl
group at C4 in order to determine its importance in biological activity. Analogs
115
-
118
were prepared as mixtures of inseparable isomers (Fig.
5.24
). The iso-
mers were moderate to poor inhibitors of MbtI, with
116
(K
i
=
290
±
90
μ
M)
and
117
(K
i
=
310
±
70
μ
M) displaying the best activity. While the C4 hydroxyl
group appeared to have little effect on activity, substitution at C3 improved the
activity against MbtI but decreased it in the case of
S. marcescens
AS, providing a
potential source of selectivity in inhibitor design.
A series of inhibitors designed to mimic the enzyme-bound isochorismate
Search WWH ::

Custom Search