Biology Reference
In-Depth Information
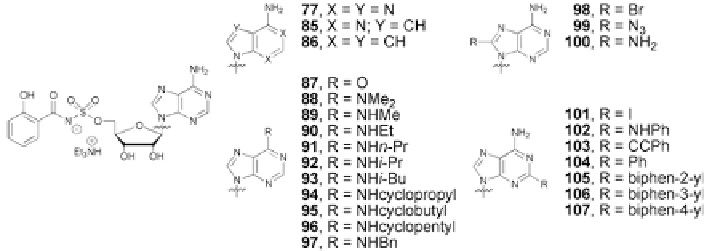
Fig. 5.21
Rationally designed nucleoside antibiotics. SAR of the base portion [
79
]
as reflected in the activity of cyclopropyl analog
94
. To analyze the analogs, a
selectivity factor
S
was defined as the ratio of inhibitory activity between iron-suf-
ficient and iron-deficient conditions. Analog
94
demonstrated to be the most prom-
ising inhibitor with an
S
value of 64 (MIC
99
=
0.098
μ
M/MIC
99
=
6.25
μ
M). The
difference between potent MtbA inhibition and whole-cell
M. tuberculosis
may
reflect the reduced permeability of these compounds where structural modification
affects the recognition and transport of the nucleosides. The analogs did not dis-
play cytotoxicity (IC
50
> 100
μ
M) and, even more remarkably, parent compound
77
did not inhibit a panel of four FadD adenylating enzymes from
M. tuberculosis
,
a demonstration of the exquisite selectivity of these nucleoside antibiotics.
5.9 Inhibition of MbtI Nonribosomal Peptide Synthetase
Work performed by Harrison et al., demonstrated that the gene Rv2386c, encoding
for enzyme salicylate synthase MbtI was essential for in vitro growth of
M. tuber-
culosis
. MbtI catalyzes the production of salicylate
109
and pyruvate
110
from
chorismate
108
(Fig.
5.22
) in the first committed step during the biosynthesis of
mycobactin T [
80
]. The crystal structure of MbtI and other chorismate-utilizing
enzymes, provided yet another target for the development of antibiotic agents target-
ing
M. tuberculosis
.
Manos-Turvey explored the synthesis of MbtI inhibitors through the syn-
thesis of chorismate analogs
111
-
113
(Fig.
5.23
). The compounds were tested
against mycobactin TI and chrosimate-utilizing enzyme
Serratia marcescens
anthranilate synthase (AS) [
81
]. In general, poor inhibition was observed against
MbtI with
111
(K
i
=
1400
±
400
μ
M), and
112
(K
i
=
3000
±
1000
μ
M),
113
(K
i
=
1700
±
500
μ
M), probably due to steric hindrance by the C5 hydroxy sub-
stituent as observed in molecular docking. However, the analogs were better inhib-
itors of
S. marcescens
AS,
111
(K
i
=
28
±
7.0
μ
M),
112
(K
i
=
90
±
14
μ
M), and
113
(K
i
=
230
±
40
μ
M). Simplified analog
114
was synthesized to assess the
effect of the hydroxyl substituents and proved to be a better inhibitor against both
enzymes MbtI (K
i
=
500
±
90
μ
M),
S. marcescen
s AS (K
i
=
3.2
±
0.3
μ
M).
Search WWH ::

Custom Search