Biology Reference
In-Depth Information
(a)
(b)
(c)
(d)
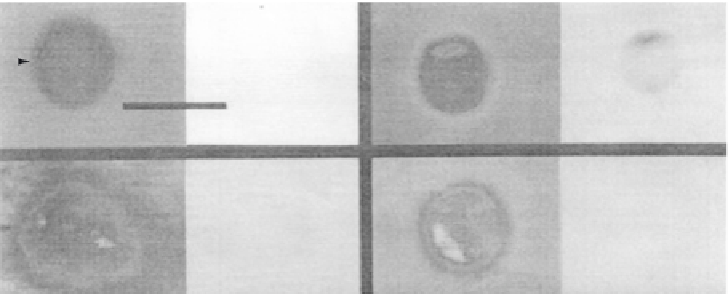
FIGURE 11.11
The cationic peripheral protein cytochrome c causes domain formation in phospholipid vesicles.
Vesicles were formed containing 1 mol% NBD PA, 4 mol% DOPA and 95 mol% DOPC. Vesicle domains were
observed by NBD fluorescence. Bar indicates 10
m
m. (a) A vesicle viewed immediately after the addition of 100
m
m
cytochrome c. Note no domains are visible. (b) A similar vesicle after 30 min in the presence of 100
m
m cytochrome c.
(c) A vesicle as in (a) viewed after 30 min in the presence of 10
m
m cytochrome c. (d) A vesicle as in (b) (domain
formation induced by 100
m
m cytochrome c) further incubated for 30 min with 0.1 M NaCl. Fluorescent domains
that are barely detectable in the presence of 10
m
m cytochrome c are obvious with 100
m
m cytochrome c and are
further augmented with 0.1 M NaCl
[41]
.
experiment also showed that the cationic peripheral protein cytochrome c causes the trans-
membrane protein gramicidin to be excluded from the PA-rich domain and to accumulate
into the PC-rich domain. Also, measurement of the cytochrome c heme absorbance demon-
strated that the peripheral protein location tracks with the PA domain. Therefore cytochrome
c causes rearrangement of both lipid and protein components of a membrane. The Haverstick
and Glaser imaging experiments are but representative examples of countless numbers of
possible lipid and protein model membrane mixtures that await testing in the future.
The most relevant example of a lipid microdomain in a biological membrane is the highly
controversial detergent-insoluble lipid raft. Lipid rafts were presented in Chapter 8 and their
methods of isolation will be discussed in Chapter 13. Rafts are an example of a lipid micro-
domain that may be partially isolated in crude form by extraction from the biological parent
plasma membrane. In reality the isolated raft is defined by a lipid composition that is a little
different than the bulk membrane, being enriched in sphingolipids and cholesterol. The raft
fractions are also enriched in characteristic signaling proteins.
Since its early development in 1982 by Binnig and Rohrer
[42]
, atomic force microscopy
(AFM) has become a major imaging technique for investigating small biological structures,
including lipid rafts. For their discovery, Binnig and Rohrer were awarded the 1986 Nobel
Prize in Physics. AFM works by dragging a small, very sharp probe across the membrane
surface while accurately measuring the vertical deflection of the probe due to changes in
surface topography (
Figure 11.12
,
[43]
)
[44]
. The probe therefore 'feels' its way across the
membrane surface allowing for accurate vertical measurements with resolution down to
<
1nm
[45]
. AFM also has the advantage of operating under water and so is applicable
for use with biological membranes under near physiological conditions.
Since lipid rafts are enriched in saturated, long chain sphingolipids and cholesterol, they
tend to be thicker than the surrounding non-raft membrane. Detecting small differences in