Agriculture Reference
In-Depth Information
CH
4
produced
from H
2
+ CO
2
CH
4
produced
from acetate
CO
2
produced
from acetate
CO
2
produced
from SOM
CO
2
consumed
by H
2
Soil
No.
16
15
12
8
6
5
3
Net surplus
or deficit of e
−
2
−
3
−
2
−
1
0
0
1
2
3
e
−
deficit (
mol g
−
1
day
−
1
)
e
−
surplus (
mol g
−
1
day
−
1
)
µ
µ
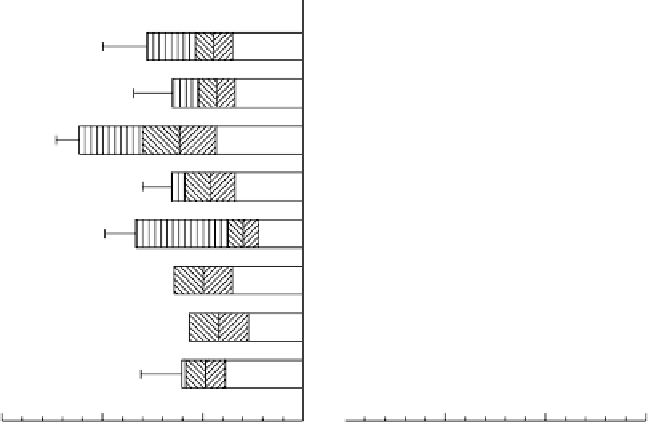
Figure 5.5
Electron balances during anoxic decomposition of soil organic matter to CH
4
and CO
2
in eight rice soils. Soil properties given in Figure 5.3 (Yao and Conrad, 2000).
Reproduced by permission of Blackwell publishing
have been acting as an electron acceptor, itself becoming reduced and allowing
more of the SOM to be oxidized to CO
2
rather than reduced to CH
4
. The latter
mechanism is consistent with the observed small proportion of CH
4
produced
from H
2
and CO
2
because an electron sink in addition to CO
2
would suppress
the concentration of H
2
.
The results imply that the average oxidation state of SOM carbon should
decrease under continuous reducing conditions. This agrees with the observed
long-term changes in the composition of SOM and accumulation of phenolic
compounds with prolonged flooding of rice soils (Chapter 3). However the field
situation differs from Yao and Conrad's experiments in that the soil receives con-
tinuing inputs of living organic matter from growing plants or other sources, with
mean oxidation state zero, and the fields are periodically drained and oxidized
for some part of the year. Therefore general conclusions cannot be drawn.
5.1.6 AEROBIC PROCESSES
The floodwater and uppermost part of the soil are oxygenated by photosynthetic
organisms, and the rhizosphere is oxygenated by leakage of O
2
from plant roots.